Buprenorphine, but not naltrexone or its extended release version Vivitrol, is associated with reduced overdose risk
Opioid-related overdose rates have increased in recent years and opioid use disorder pharmacotherapies like naltrexone, its extended release version (i.e., Vivitrol), and buprenorphine (i.e., Suboxone when paired with naloxone) have the potential to reduce risk of overdose. It is unclear if certain pharmacotherapies are more or less effective in preventing overdose and whether their effectiveness varies as one stops taking the medication. This study suggests that being on buprenorphine is effective for reducing overdose risk, and perhaps more effective than naltrexone, but only while undergoing active treatment.
WHAT PROBLEM DOES THIS STUDY ADDRESS?
The United States has experienced a significant increase in opioid use disorder and opioid-related overdose since the 1990’s, with over 130 deaths occurring daily. Medications for opioid use disorder help reduce opioid use and risk for overdose, while improving the likelihood that people stay in treatment (i.e., treatment retention). Opioid antagonist medications, like naltrexone, work by blocking the effects of opioids, whereas opioid agonist medications, like Suboxone, provide stable low–level stimulation of opioid receptors and block the effects of other opioids (e.g., heroin). Discontinuation of these medications is relatively common and, particularly for opioid antagonists, might be followed by a period of increased overdose risk. Reducing opioid use during treatment lowers one’s tolerance to the effects of opioids, so returning to opioid use in large quantities after discontinuing medication treatment can result in overdose. However, it is unclear whether overdose risk during treatment and following discontinuation is different between naltrexone and buprenorphine. In the current study, authors used a large database of commercially insured adults to examine the real-world effectiveness of these medications in reducing opioid-related overdose.
HOW WAS THIS STUDY CONDUCTED?
The authors conducted a naturalistic observational study of opioid-related overdose data from the Truven Health Analytics MarketScan Commercial Claims Database (MarketScan), which included medical claims for pharmacy, diagnostic testing, ambulatory, and inpatient visits among a representative sample of the commercially insured U.S. population. Data were collected between the years 2010 and 2016. The study sample included 46,566 individuals (average age of 33; 62% male) with a diagnosis of opioid use disorder (determined with the International Classification of Diseases, Ninth Revision or Tenth Revision (ICD-9 or ICD-10)), who had initiated either buprenorphine alone or in combination with naloxone (referred to here for convenience by its common brand name, Suboxone), oral naltrexone, or extended-release injectable naltrexone (referred to here for convenience by its common brand name, Vivitrol).
Treatment initiation was defined as the earliest recorded date of the patient filling a prescription for Suboxone or naltrexone. Treatment coverage and discontinuation was defined using the date that the prescription was filled, and the number of days covered by the prescription. Discontinuation was defined as a given 4-week window without any medication following a recent period of continuous medication prescription. Because individuals can fluctuate being on and off a given medication over time, the authors analyzed periods of time spent on/off a particular medication, rather than the individual participants themselves. Analyses therefore assessed the likelihood of overdose while on a medication vs. when medication was recently discontinued (4 weeks without medication) vs. being off of the medication beyond 4 weeks. Overdose (fatal and non-fatal) was identified via inpatient and outpatient medical claims of overdose. The authors calculated the incidence of overdose per 100 person years (i.e., the number of overdoses divided by the total number of years that the data covers added across each person, which is then expressed as a fraction of 100). 1,805 individuals experienced an opioid-related overdose during the study period. Accounting for individuals who had more than one overdose, a total of 2,755 overdose events were reported (2,020 overdose events while individuals were not on treatment, 620 overdose events while on Suboxone, 100 overdose events while on naltrexone, and 15 overdose events while on Vivitrol).
Suboxone was the most commonly prescribed pharmacotherapy (received by 84% of participants), followed by naltrexone (14%) and Vivitrol (2%). Participants had an average of 1.5 years of follow-up data, and 83% received other types of prescription medications (e.g., benzodiazepines) during the study period. Co-occurring substance use disorders included alcohol (22%), sedative (13%), cannabis (12%), cocaine (8%), amphetamine (6%), and hallucinogen (<1%) use disorders. The study sample primarily consisted of individuals with PPO (preferred provider organization) insurance coverage (61%), who were either the primary beneficiary (insurance provided by an employer; 38%) or the dependents/children of a primary beneficiary (40%).
WHAT DID THIS STUDY FIND?
Overdose incidence and risk were significantly lower during periods of active Suboxone treatment.
Compared to periods spent without any medication treatment (4.98 overdoses per 100 person years), overdose incidence during Suboxone treatment was significantly lower (2.08 overdoses per 100 person years). Overdose rates during Vivitrol treatment (3.85 overdoses per 100 person years) and oral naltrexone treatment (6.18 overdoses per 100 person years) did not significantly differ from rates during periods without any pharmacotherapy (4.98 overdoses per 100 person years).
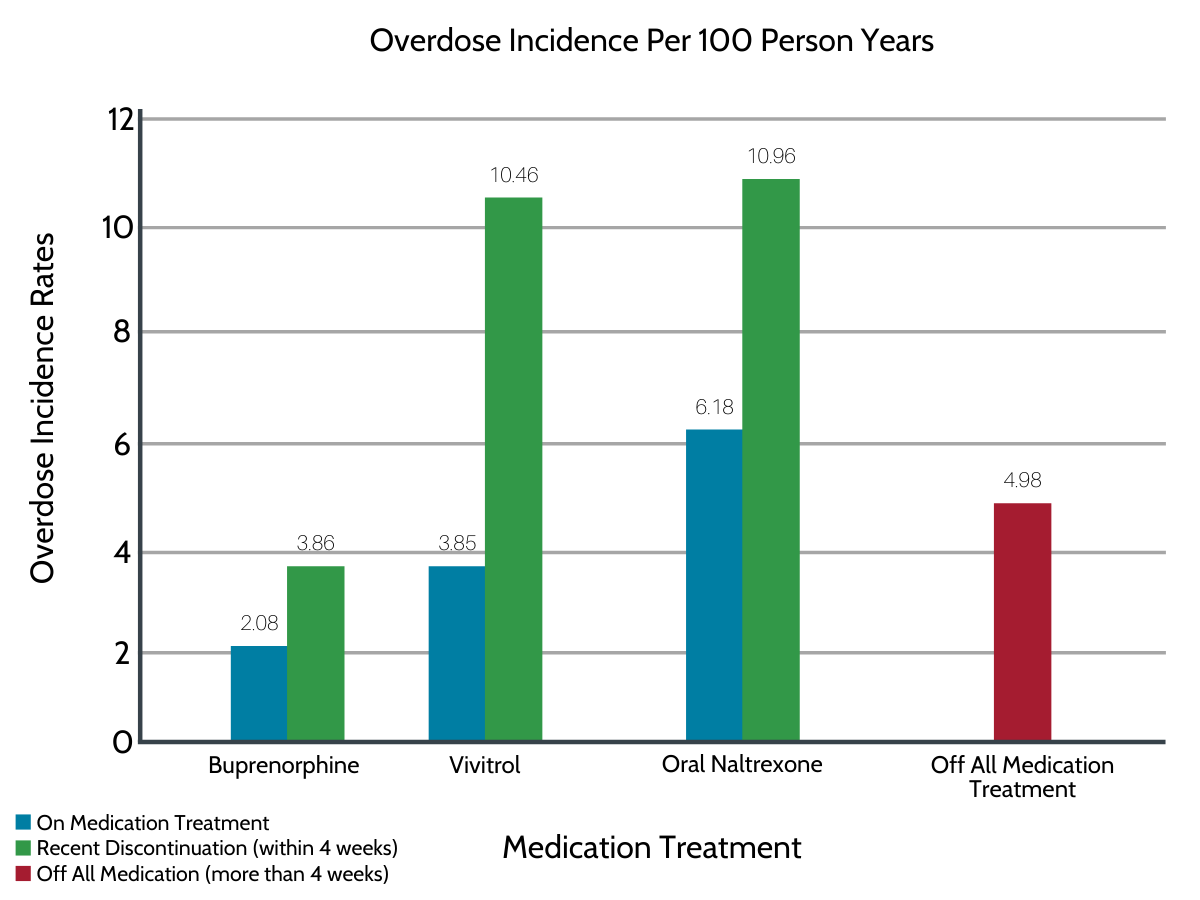
Figure 1. Controlling for demographics (age, sex, geographic region) and clinical factors (type of insurance coverage, co-occurring substance use disorders, other prescription medications, clinician visits), Suboxone was the only treatment associated with lower risk of overdose compared to periods of no medication treatment.
Risk of overdose did not differ between periods of no treatment and periods following recent discontinuation by medicine type.
In absolute terms, overdose incidence rates (per 100 person years) within 4 weeks of discontinuing treatment were lowest for Suboxone (3.86 overdoses per 100 person years) and overdose incidence was higher following discontinuation of naltrexone (~10 overdoses per 100 person years). When controlling for participant characteristics, however, risk of overdose during periods of no treatment did not significantly differ from risk of overdose during periods of recent Suboxone and naltrexone discontinuation.
There were other key demographic and clinical factors associated with an increased risk of overdose.
Receipt of other prescription medications (e.g., benzodiazepines like alprazolam, known commonly by its brand name Xanax), receiving services at an addiction treatment facility, diagnosis of a co-occurring substance use disorder (e.g., alcohol use disorder), younger age, being a child/dependent on the insurance policy of a primary beneficiary, and living in the Northeast or Midwest of the United States were each uniquely associated with greater likelihood of overdose. That is, when controlling statistically for whether or not someone was taking medication, as well as the other factors in the model, each of these variables was related to greater overdose risk.
WHAT ARE THE IMPLICATIONS OF THE STUDY FINDINGS?
This study suggests that, when compared to periods of no-medication treatment, Suboxone appears to be effective at reducing risk of opioid related overdose in a nationally representative sample of commercially insured individuals with opioid use disorder started on Suboxone (or buprenorphine alone), naltrexone, or Vivitrol.
Outcomes add to existing data on overdose reductions among individuals receiving opioid agonist therapy. Other studies have shown that Suboxone confers greater benefit earlier in treatment because it is more difficult to get individuals started on Vivitrol. Treatment outcomes (24 weeks), however, including overdose events, have shown to be similar for individuals who successfully start these medications. Though this study’s findings suggest Suboxone may be more effective at preventing overdose than naltrexone and Vivitrol during periods of active treatment, cumulative time spent on naltrexone and Vivitrol, and total number of overdose events while taking these medications was relatively small, and this has the potential to mask any potential benefits. Nonetheless, Suboxone’s demonstrable ability to reduce overdose risk supports harm-reduction arguments for making it available without a prescription in emergency situations. Expanding access to Suboxone and encouraging retention in medication treatment will not only facilitate reduced opioid use but is also likely to help lower exceptionally high rates of opioid-related overdose.
Overdose risk was not significantly affected by recent discontinuation of either Suboxone or naltrexone, suggesting that overdose risk may remain constant while off the medication, regardless of the recency of the treatment episode and type of medication. However, this finding requires additional investigation, given the study’s reduced ability to detect naltrexone’s effects.
Naltrexone’s antagonist actions raise particular concern that reduced opioid tolerance might intensify risk of overdose upon stopping naltrexone treatment. Despite the non-significant outcomes of this study, the incidence of overdose was two times higher upon recent discontinuation of naltrexone, compared to periods of no medication treatment. The additional analysis conducted that controlled for other clinical characteristics suggested that this higher incidence of overdose on discontinuation of naltrexone was explained potentially by these other factors. More research is needed to clarify overdose risk in the context of medication discontinuation to understand more fully what specifically increases risk and who is more at–risk. Of the 70,000 person years under investigation, all of which were in individuals started on some medication, 40,000+ years were spent off medication (which did not include the 4–week discontinuation window). Therefore, it is important to better understand why individuals are stopping medication treatment in order to inform ways to prevent discontinuation or support successful transition off the medication. It is also important for future investigations to include individuals who have never taken opioid use disorder medication, given that all participants in this study had exposure to medication which may influence outcomes.
This study also reveals the importance of several other factors in the risk of opioid-related overdose. Co-occurring substance use disorders, receipt of other prescription medications (e.g., benzodiazepines), and visiting an addiction treatment facility were all associated with increased risk of overdose, suggesting that populations presenting with more severe substance use disorder histories may require additional support to prevent overdose incidence. The use of other prescription medications with sedative effects is particularly important with respect to opioid agonist therapies, given that the combined effects of these medications can dangerously suppress the central nervous system and contribute to overdose (e.g., when Suboxone is taken with benzodiazepines and/or alcohol). Findings also suggest that younger individuals may be at increased risk of overdose, which is consistent with national data showing the highest overdose rates among individuals under the age of 50. National data also suggest higher rates of overdose among men and recently released prison inmates, highlighting the role of a variety of factors in overdose risk. Clarifying the influence of these factors and their interaction will ultimately help to better identify and assist high risk individuals and prevent overdose.
- LIMITATIONS
-
- Participants in this study were commercially insured and may not be representative of the broader population. Time spent during active naltrexone treatment was relatively short, the number of overdose events while on naltrexone was small, and only 2% of individuals received a Vivitrol prescription in this database, which may have limited power to detect naltrexone’s effects and warrants additional research.
- Overdose that did not result in a medical claim was not captured here and overdose events for which individuals did not seek medical care are unaccounted for in this study. Furthermore, this study could not differentiate between fatal and non-fatal overdose. The authors defined current medication treatment as filling a prescription and this measure does not capture dose or adherence to the medication, which could influence overdose rates.
- Naltrexone’s and Suboxone’s effectiveness were assessed by comparing them to periods without medication treatment. Given that all participants had exposure to opioid use disorder pharmacotherapy and this exposure may confer benefits lasting beyond the treatment episode, investigations that incorporate those who had never taken opioid use disorder medication (i.e., pharmacotherapy naïve individuals) are needed.
BOTTOM LINE
- For individuals and families seeking recovery: This study suggests that buprenorphine, often prescribed in combination with naloxone and commonly known by the brand name Suboxone, can help to prevent opioid-related overdose. Given that periods of active treatment were compared to periods when medication was no longer being received, findings suggest that retention in buprenorphine treatment may be key to reducing risk of overdose. Naltrexone and its extended release version, commonly known by the brand name Vivitrol, may not be as effective at preventing overdose and individuals taking these medications may therefore need additional support to reduce overdose risk. However, additional research is needed before forming conclusions about naltrexone’s effects on opioid-related overdose. Still, this study provides evidence of Suboxone’s ability to help prevent overdose and lends insight to populations that might be at greater risk for opioid-related overdose (e.g., younger individuals with co-occurring substance use disorders). Further characterization of these risk factors and protective mechanisms will ultimately help guide the best approaches to prevent overdose.
- For treatment professionals and treatment systems: This study suggests that pharmacotherapy patients may be at increased risk of opioid-related overdose when the patient is no longer undergoing treatment with buprenorphine, often prescribed in combination with naloxone and commonly known by the brand name Suboxone. It also suggests that naltrexone and its extended release version, commonly known by the brand name Vivitrol, may not be as effective at preventing overdose, but more research is needed given the limitations of the database investigated. Pharmacotherapy patients who are younger, prescribed other medications, seeking other addiction treatment services, and have co-occurring substance use disorders may be at increased risk for overdose. Additional treatment/recovery resources may be needed to help support those with a higher risk of opioid-related overdose. Patients with low rates of Suboxone retention, and possibly naltrexone/Vivitrol patients, may also require extra support to prevent overdose.
- For scientists: This study found that pharmacotherapy patients had a reduced risk of opioid-related overdose when undergoing buprenorphine treatment, compared to periods of no medication receipt. Naltrexone, on the other hand, did not significantly affect overdose risk. Overdose risk was also not significantly affected by recency of pharmacotherapy discontinuation. Given the importance of understanding naltrexone’s benefits/risks, particularly with regard to overdose following loss of opioid tolerance and medication discontinuation, additional research is needed to evaluate these outcomes in a population with more person-time accumulated on naltrexone. Prospective, as opposed to retrospective, research will ultimately improve our understanding of overdose risk as it relates to opioid use disorder pharmacotherapy.
- For policy makers: Studies like this help us to identify predictors of opioid-related overdose as it relates to opioid use disorder pharmacotherapy. Given that opioid-related overdose constitutes a nationwide epidemic and opioid use disorder medications (i.e. oral naltrexone, Vivitrol, and Suboxone) are effective and necessary, it is essential to identify overdose risk throughout the course of medication treatment and discontinuation. The current study suggests that Suboxone might prevent opioid overdose and pharmacotherapy patients may be at increased risk of opioid-related overdose when the patient is no longer taking it. Although naltrexone and Vivitrol were not associated with reduced overdose risk, the number of overdose events while on these medications was small, which may have limited power to detect their effects and demands additional research. Funding is needed to conduct prospective, rather than retrospective, research on overdose risk and protective mechanisms, which will ultimately help guide approaches to address high rates of opioid-related overdose in the United States.
CITATIONS
Morgan, J. R., Schackman, B. R., Weinstein, Z. M., Walley, A. Y., & Linas, B. P. (2019). Overdose following initiation of naltrexone and buprenorphine medication treatment for opioid use disorder in a United States commercially insured cohort. Drug and alcohol dependence, 200, 34-39. doi: 10.1016/j.drugalcdep.2019.02.031

