Suboxone vs. Vivitrol: A Head-to-Head Comparison
While both Suboxone and Vivitrol outperform placebo medication, this critically important study pitted these two commonly prescribed medications against each other.
WHAT PROBLEM DOES THIS STUDY ADDRESS?
Opioid overdoses alone accounted for 33,000 overdoses in the United States in 2015. Overdose (including but not limited to opioids), is now the leading cause of accidental death in the U.S., identifying an urgent need to identify effective treatments for opioid use disorder.
SUBOXONE
Buprenorphine, often prescribed in a formulation with naloxone (brand name Suboxone), is an opioid agonist that can help reduce opioid use or protect against relapse both over the short-term as well as the long-term, compared to no medication. Naturalistic research also suggests Suboxone helps reduce the likelihood of overdose death.
- DRAWBACKS & CONCERNS
-
- First, Suboxone is better than a placebo medication, but there is room for improvement. For example, Suboxone patients in rigorous studies still produce up to 60% opioid positive urine toxicology screens (“tox” screens), with better outcomes for those who only misuse pharmaceutical opioids.
- Second, like most medications, an individual needs to take buprenorphine on a daily basis – which can be problematic for individuals with substance use disorder given that motivation can be variable especially early in the recovery process.
- Third, some clinicians have concerns about prescribing an opioid agonist like buprenorphine to those who are still drinking alcohol or using benzodiazepines, since they are also central nervous system depressants and, in combination, can have health consequences including overdose.
VIVITROL
Another evidence-based medication for opioid use disorder is naltrexone, an opioid antagonist formulated as an extended-release once-a-month injection (referred to here as the brand name Vivitrol). This medication is delivered once every 4 weeks, instead of everyday and might help address concerns about needing to take the medication daily as well as the potential problems that could arise if someone is also taking other drugs (in addition to opioids) like alcohol.
- DRAWBACKS & CONCERNS
-
- One of the main concerns about naltrexone is that because it is an opioid antagonist (i.e., blocker), an individual needs to be abstinent from opioids for at least a few days so that they do not go into immediate withdrawal when administered the medication. For some individuals, this need to not use any opioids before receiving Vivitrol can be very difficult and uncomfortable, and thus can lead to resumption of illicit opioid use making this transition a clinical challenge.
HOW WAS THIS STUDY CONDUCTED?
In this study, Lee and colleagues conducting a critically important study specifically comparing Suboxone to Vivitrol. Authors used a randomized controlled trial to test Suboxone against Vivitrol in 570 individuals (287 receiving Suboxone and 283 receiving vivitrol) for 6 months who were attending one of eight inpatient detoxification programs in the U.S. The primary outcome was relapse, and the secondary outcomes were: failure to initiate the medication, percent of days using an opioid during the 6-month study, and adverse events including overdoses.
- MORE ON STUDY METHODS
-
All participants had opioid use disorder according to the diagnostic and statistical manual of mental disorders, 5th edition (DSM-5) and had used opioids at least one of the past 30 days.
While the sites differed in terms of how they helped individuals medically withdraw from opioids (“detox”), all were short-term (3-7 days) and part of a community program that provided at least weekly therapy sessions (group or individual) on an outpatient basis during the study. The study was conducted as part of the National Institute on Drug Abuse’s Clinical Trials Network, which tests evidence-based treatments for substance use disorder in community settings. Of note, study site and severity in terms of opioid use were taken into account when randomizing patients to receive to Suboxone vs. Vivitrol.
All patients received medical management at each study visit including psychoeducation about opioid use disorder, an adherence plan, advice to abstain from all substance use, monitoring of side effects, and encouraging attendance at therapy and mutual-help groups. The medical management schedule was the same for both Suboxone vs. Vivitrol – weekly for the first month, then every two weeks for the next 3 months, then every month for the final 2 months. Suboxone doses ranged from 8 to 24 mg depending on clinical need – these are typical doses. Vivitrol was administered every 28 days, and before starting the medication, individuals needed to be abstinent from all opioids for at least 3 days, have a toxicology screen negative for opioids, and pass a “naloxone challenge” to be sure they would not withdraw from the Vivitrol.
The primary outcome was “relapse”, measured after the first 3 weeks, and defined as one of the following:
- 4 consecutive weeks of opioid use determined by urine drug test (a missed drug test was counted as “positive” indicating use)
- 4 consecutive weeks of opioid use determined by self-report
- 7 consecutive days of self-reported opioid use
CHARACTERISTICS OF STUDY SAMPLE
Important characteristics of the study sample were that they were 34 years old, on average, 70% male, 75% White and 10% Black and 13-20% Latino. Most (80%) were heroin users, more than 60% were injection drug users, and 35-40% had prior treatment. Other substance use in the past 30 days was common: 47-57% with stimulant use, 25-32% with sedative use, 42-48% with cannabis use, and 25-27% with “heavy” drinking, which was not defined by authors but, according to the Substance Abuse and Mental Health Services Administration (SAMHSA), means 5 or more drinks for men or 4 or more for women on the same occasion at least 5 days in the past month. Nearly 70% reported a lifetime history of another psychiatric disorder, and they had “mild” depressive symptoms, on average, measured by the Hamilton Depression Scale. Individuals were not able to participate in the study if they had “serious medical, psychiatric, or substance use disorder” as determined by the study physician.
WHAT DID THIS STUDY FIND?
In the Suboxone group, 57% relapsed during the study, significantly less than the 65% who relapsed in the Vivitrol group (i.e., unlikely due to chance – a reliably large difference).
As seen in the figure below:
- On average Suboxone individuals had 10 negative (opioid-free) tox screens, while Vivitrol individuals only had four negative (opioid-free) tox screens.
- Out of 144 days, Suboxone individuals reported 81 opioid abstinent days and Vivitrol 39 abstinent days.
- The Suboxone group had a significantly longer time until they relapsed, on average (14 weeks vs. 8 weeks).
- In any given week, if one person was abstinent in the Suboxone group and a similar person was abstinent in the Vivitrol group, the person in the Suboxone group had a 36% higher odds of avoiding relapse that week.
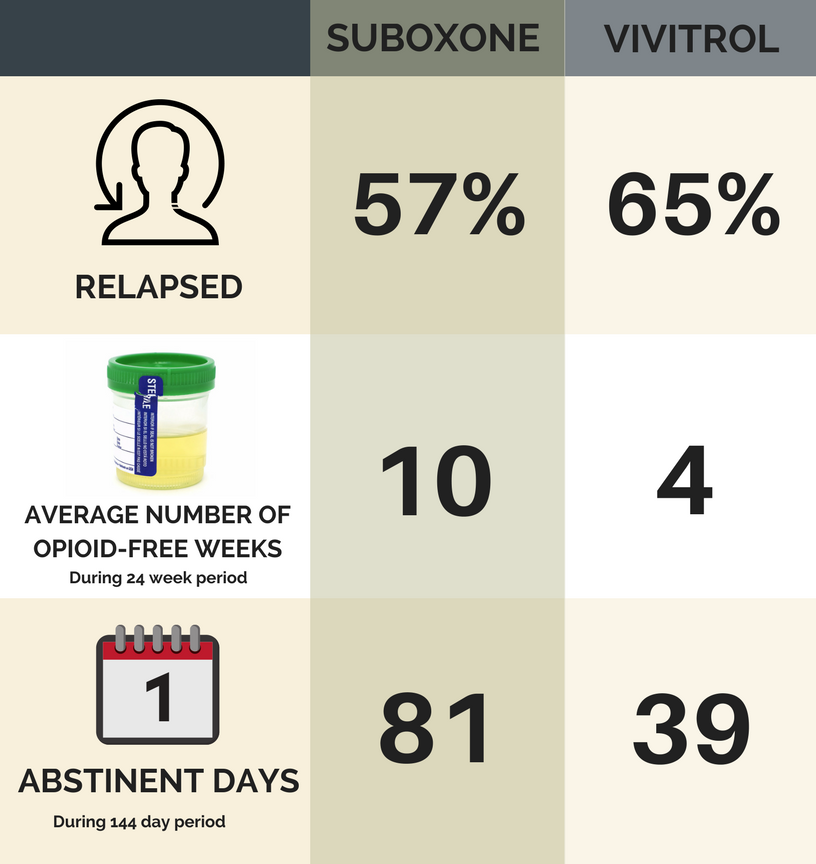
*as verified by urine toxicology screen
Noteworthy from this study is that the advantage of Suboxone occurred early – in the first 6 weeks. For those who make it past 6 weeks, Vivitrol begins to show an advantage.
This benefit of Suboxone is primarily accounted for by the difficulty getting individuals started on Vivitrol – which is consistent with anecdotal clinical observations and concerns. The study revealed difficulty getting started on Vivitrol – 94% of individuals assigned to Suboxone were successfully started (e.g. induction) while only 72% of those assigned to Vivitrol were successfully started on the medication. When only individuals successfully started on the medications were analyzed, the Suboxone and Vivitrol outcomes were similar.
Vivitrol individuals were more likely to be successfully started if beginning the medication more than 3 days after their last use of opioids. Having at least a few days abstinent made a difference for Vivitrol patients.
Adverse events – including overdose – were not different between the groups. More specifically, 15 assigned to Vivitrol had an overdose (two fatal) and eight assigned to Suboxone had an overdose (three fatal). In other words, 23 of the 570 patients in the entire trial had an overdose, five of whom died, during the study period – less than 1%.
The overall “success” rates for this study were modest, with more than half of all participants relapsing in the first 6 months of this rigorously delivered, evidence-based trial, suggesting there remains much room for improvement.
- RELATED STUDY
-
In a related study taking place in Norway that also compared Suboxone to Vivitrol, all patients had already detoxified from opioids – essentially removing any influence of needing to establish a period of abstinence to get started on Vivitrol, because it was a constant. Not surprisingly, in that study Vivitrol did as well, or in some cases, better than Suboxone. So, in settings where individuals are safely detoxified and time is not a factor (e.g., in the Veterans Administration), Vivitrol may be an equally effective option at least in the short term. On the other hand, in outpatient settings or when detoxification periods are relatively rigid – more common in addiction treatment settings – Suboxone appears to be the better option.
- LIMITATIONS
-
- There was no group receiving a placebo medication to which to compare Suboxone and Vivitrol – however, the efficacy of each medication compared to placebo is well established.
- All individuals were recruited from an inpatient detoxification program while the primary draw of each medication is that they can be initiated and prescribed on an outpatient basis.
- Individuals with “serious” other psychiatric disorders – – were excluded. This decision on who to exclude was made by a study physician. Also since patients were recruited from an inpatient detoxification program, they may be more motivated for change than your average patient. This introduces two concerns. First, whether the findings from this study can be applied to the population of individuals with opioid use disorder is not clear. Second, those with more psychiatric comorbidities could have greater difficulty with day-to-day tasks, including but not limited to taking a medication. So, daily medication use may be a greater challenge, which might favor Vivitrol which is administered once every 4 weeks. Also, an agonist treatment with some – albeit minimal – potential for misuse may be more of a concern for those with other substance use disorders. Given the study design, it is not possible to answer many of these questions but they are important to applying study results and informing future work.
BOTTOM LINE
- For individuals & families seeking recovery: If you or your loved one has an opioid use disorder, Suboxone and Vivitrol both may help reduce craving and protect against relapse. Vivitrol, however, may be harder to start as it requires a period of at least a few days of opioid abstinence. These medications are not cure-alls – more than half of individuals relapsed during this 6-month study, even in the highly rigorous and controlled circumstances of a clinical trial.
- For scientists: In this multisite randomized controlled trial Suboxone outperformed Vivitrol, primarily because more individuals assigned to Suboxone were able to get started on the medication right away. We need additional investigations into medication moderators, as well as how to boost medication outcomes given that even in this highly rigorous trial, more than half of participants relapsed.
- For policy makers: Suboxone and Vivitrol both help reduce craving and protect against relapse. Vivitrol, however, may be harder to start as it requires a period of at least a few days of opioid abstinence. While these medications are not cure-alls – more than half of individuals relapsed during the 6 month trial, even in the highly rigorous and controlled clinical trial, policies to increase the dissemination of these evidence-based medications would still likely decrease the health, criminal justice, and financial burdens attributable to the opioid epidemic. Funding for research on other types of clinical and recovery support services is greatly needed to further improve individual outcomes.
- For treatment professionals and treatment systems: Suboxone and Vivitrol both may help reduce craving and protect against relapse. Vivitrol, however, may be harder to start as it requires a period of at least a few days of opioid abstinence. If choosing between one or the other to reduce opioid use: Suboxone is the better choice in outpatient settings, while Suboxone and Vivitrol will fare similarly, on average, in an inpatient setting, where programs have the benefit of an extended detoxification if needed. These medications are not cure-alls – more than half of individuals relapsed during the 6 month trial, so more research is needed on clinical and recovery support services that can be integrated with these medications to improve outcomes.
CITATIONS
Lee, J. D., Nunes, E. V., Novo, P., Bachrach, K., Bailey, G. L., Bhatt, S., … & King, J. (2017). Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X: BOT): a multicentre, open-label, randomised controlled trial. The Lancet.
READ MORE ABOUT MEDICATIONS & PHARMACOLOGY
