Mixing meds: Weighing the pros and cons of adding benzodiazepines to buprenorphine treatment for individuals with opioid use disorder
Benzodiazepines (a class of sedative medications best known by brand names like Valium, Xanax, and Klonopin) may be prescribed to patients who are receiving buprenorphine treatment for opioid use disorder (an opioid agonist medication best known in formulation with naloxone, marketed as Suboxone). Clinicians accept that there are both risks, and potential benefits associated with combining these medications. To help aid clinical recommendations, Park and colleagues examined some of the risks and benefits associated with prescribing buprenorphine and benzodiazepines together using government records from the state of Massachusetts.
WHAT PROBLEM DOES THIS STUDY ADDRESS?
Benzodiazepines are commonly prescribed to manage anxiety and insomnia in patients receiving buprenorphine treatment for opioid use disorder, with approximately a third of patients taking buprenorphine also being prescribed benzodiazepines, and up to another third regularly using benzodiazepines obtained illicitly. Though it is thought that combining these medications may help some individuals stay engaged in treatment and reduce risk for return to opioid use because their anxiety and/or sleep issues are being managed, combining these medications also confers significant risk. This is because combining benzodiazepines with other medications with sedating effects (such as buprenorphine) can substantially increase risk for overdose by increasing respiratory depression, leading to hypoxia (a condition in which the brain is damaged by oxygen deprivation) and eventually death.
Though some studies have spoken to the potential benefits of combining these medications to treat opioid use disorder, showing for instance increased treatment retention, and that combining these medications does not significantly increase risk of drug overdose (see Abrahamsson et al., and Dupouy et al.), sample sizes in many of these studies have been relatively small. Studies with large sample sizes that can control statistically for the overall impact of co-occurring psychiatric disorders and other medications, could aid practitioners when faced with these critical decisions regarding prescription of multiple sedating medications.
Thus, the authors of this large, rigorous study of Massachusetts residents aimed to assess whether: 1) benzodiazepine prescribing during buprenorphine treatment is associated with increased risks of fatal and non-fatal opioid overdose or death from other causes, and 2) benzodiazepine prescribing during buprenorphine treatment is associated with reduced risk of buprenorphine discontinuation (i.e., leaving treatment).
HOW WAS THIS STUDY CONDUCTED?
This was a retrospective cohort study of 63,345 people aged 18 years or older who received buprenorphine treatment while residing in Massachusetts between January 2012 and December 2015. The observation time for all people started on the date of the first filled buprenorphine prescription during the study period. Individuals were only included if they did not have a buprenorphine prescription filled during the year prior to the start of observation. The potential minimum and maximum periods of observation were one day and four years. Among the study sample, 15,283 people (24%) had filled at least one benzodiazepine prescription during buprenorphine treatment. Study outcomes included: 1) fatal opioid overdose, 2) non-fatal opioid overdose, 3) death by other cause, and 4) buprenorphine treatment discontinuation defined as having a 30-day gap without another prescription following the end date of their previous prescription. The authors tried to isolate the effect of the potential influence of benzodiazepines by statistically controlling for a number of additional individual factors that could also have influenced study outcomes including: age, sex, Medicaid insurance status, presence of co-occurring psychiatric disorders (e.g., anxiety, depressive, bipolar, or psychotic disorders), prescription of anti-depressant medications, whether the individual had any treatment encounters with a medical claim containing a mental health diagnosis code, and buprenorphine daily dose.
The authors utilized data from the Massachusetts Public Health Data Warehouse, which combines multiple state government datasets to better understand opioid-related harms in Massachusetts. This data set includes the Massachusetts All-Payer Claims Database, which contains health and pharmacy insurance claims data from private and public health-care payers (representing around 98% of Massachusetts residents). Other data sources included the Prescription Monitoring Program, the Registry of Vital Records and Statistics death records, the Acute Care Hospital Case Mix tracking hospital visits, and the Massachusetts Ambulance Trip Record Information System.
For the purposes of the study, the authors equated filling a prescription for buprenorphine or benzodiazepines with actually taking these medications, though they recognize this may not have always been the case.
A sophisticated analytic approach was utilized that measured the effects of exposure to benzodiazepines (represented by a benzodiazepine prescription) on fatal opioid overdose, non-fatal opioid overdose, death by other cause, and buprenorphine treatment discontinuation.
WHAT DID THIS STUDY FIND?
Those who received benzodiazepines were more likely to be female (49% of those who received a benzodiazepine were female versus 34% of those who did not), have an anxiety disorder diagnosis (36% versus 18%), depressive disorder (40% versus 23%), bipolar or psychotic disorder (11% versus 6%), have had a recent hospital based mental health-related encounter (41% versus 35%), and were older (mean age= 40 versus mean age= 37 years).
During the four-year study period, of those treated with buprenorphine, 183 people died of an opioid overdose. There were also 693 non-fatal opioid overdoses and 369 people died from any other cause. Approximately 31% of fatal opioid overdoses occurred during times when people received benzodiazepines during buprenorphine treatment.
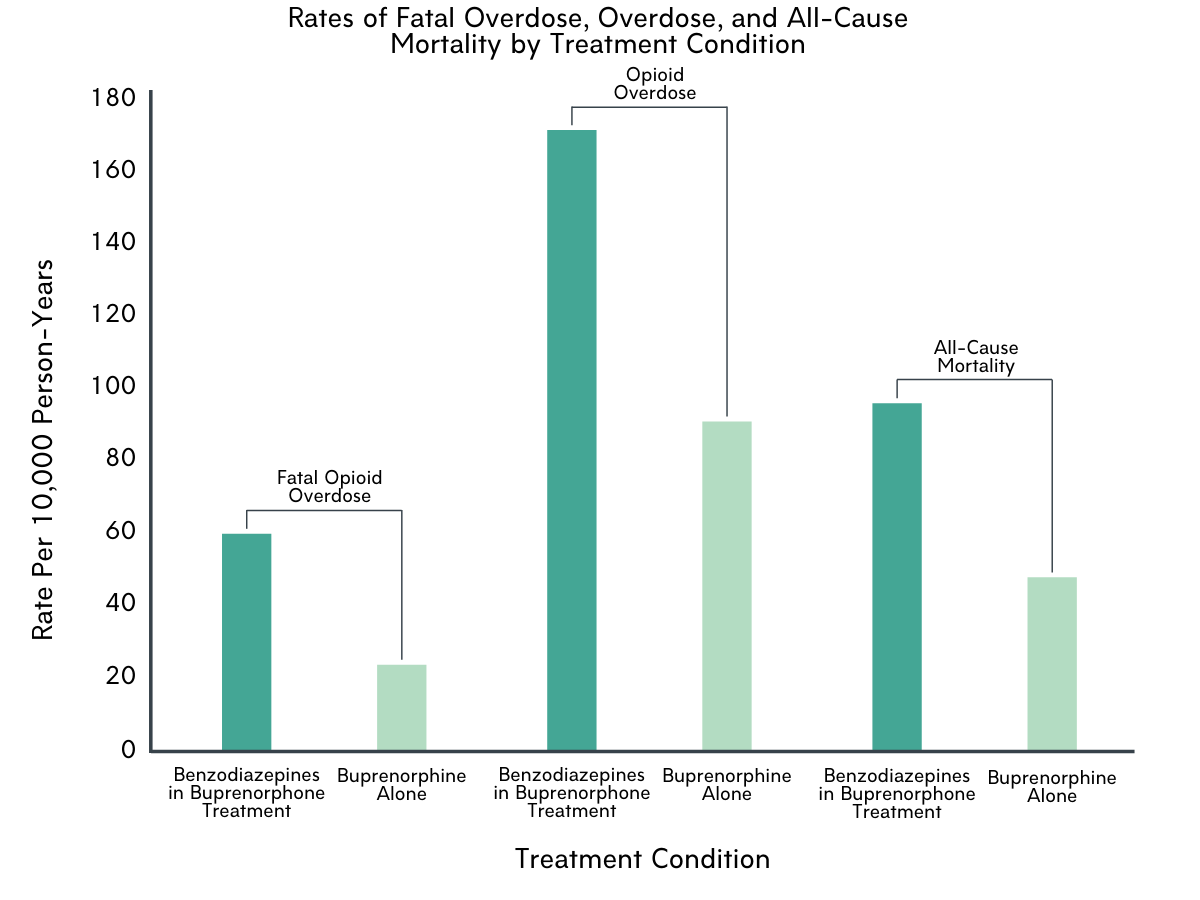
Figure 1. Rate of fatal overdose, overdose, and all-cause death by treatment condition. The vertical axis represents rate per 10,000 person-years. Person-years are calculated as the total number of people in the study multiplied by the total amount of time participants are in the study. This is a common way epidemiologists represent the frequency of an event over a specified period of time.
Compared to periods during which people received buprenorphine alone, periods of concurrent benzodiazepine and buprenorphine receipt were associated with almost three times the likelihood of experiencing opioid-related overdose death, adjusting statistically for the variables mentioned above like diagnosis of co-occurring psychiatric disorders and taking anti-depressant medication. Benzodiazepine treatment during buprenorphine treatment was also associated with approximately twice the risk of non-fatal opioid overdose and death from all causes. At the same time, benzodiazepine treatment during buprenorphine treatment was associated with a slightly decreased risk of buprenorphine treatment discontinuation.

Figure 2.
WHAT ARE THE IMPLICATIONS OF THE STUDY FINDINGS?
Fatal opioid overdose during buprenorphine treatment was relatively rare. Among the 63,345 individuals who received a buprenorphine prescription in the study, there were only 183 fatal opioid overdoses during the four-year study period, representing fewer than 4% of the 4754 estimated total opioid overdoses in the state of Massachusetts. This finding reinforces previous research demonstrating the life-saving benefits of buprenorphine.
In this study, receipt of benzodiazepines during buprenorphine treatment was associated with an increased risk of overdose and death from all causes. The authors speculate that because benzodiazepines can increase respiratory depression, especially when combined with other sedating medications like buprenorphine, benzodiazepine use may increase the risk of overdose. Additionally, given that patients receiving methadone treatment for addiction sometimes take benzodiazepines ‘to feel good’ or ‘to get high’, or to ‘boost’ the effect of methadone, benzodiazepine use in the setting of buprenorphine treatment may increase the risk of overdose by causing overuse of benzodiazepines or by inducing relapse to illicit opioid use. While authors also mention the possibility that benzodiazepine prescription is simply a marker for more severe psychological symptoms, which themselves may place individuals at greater risk for relapse, their analyses controlled for anxiety and mood disorders and associated medications, making this alternative explanation for increased mortality risk in the benzodiazepine group possibly less plausible.
Though combining benzodiazepines with buprenorphine appears to increase risk for overdose and death, it also appears to decrease risk of buprenorphine treatment discontinuation. It is possible that benzodiazepine treatment may lead to improved buprenorphine treatment retention because it can effectively treat factors like anxiety and sleep disturbance that can lead to relapse. Additionally, the authors observed that former benzodiazepine receipt (i.e., receiving benzodiazepines prior to, but not during the study period) was actually associated with an increased risk of buprenorphine discontinuation. It’s possible that people formerly, but not actively prescribed benzodiazepines have under-treated of anxiety and insomnia, leading to worse buprenorphine treatment adherence.
It has previously been shown that having a psychiatric diagnosis in addition to opioid use disorder among those receiving buprenorphine is associated with improved treatment retention. It is possible that this may have influenced this finding, such that those with co-occurring mental disorders who are inherently more likely to stay engaged in treatment, also happen to be more likely to receive benzodiazepines. Said another way, it may be the co-occurring psychological disorder/s that is leading individuals to seek and remain in buprenorphine treatment, rather than the benzodiazepine prescription itself. Alternatively, it may be that benzodiazepine use mediates the relationship between co-occurring psychological disorders and increased treatment retention.
It is important to note here that the first-line treatments for anxiety and sleep disorders are cognitive-behavioral interventions. Combining non-pharmacological approaches like cognitive behavioral therapy with buprenorphine treatment would be a safer, and probably more effective way to manage these co-occurring issues.
- LIMITATIONS
-
As noted by the authors:
- It was assumed that individuals took their medications according to prescribers’ instructions, though in reality they may have not. This could have biased the results in unknown ways.
- Massachusetts prescription records show fill dates, not collection dates. Thus, there may have been a lag between the fill date and the date the patient collected the medication. This could also have biased the results in unknown ways.
- Because of the observational nature of this study’s design, it cannot be known whether benzodiazepine use is a direct cause of overdose, death or increased buprenorphine treatment retention.
- Toxicology data at the time of death was not included in the authors’ analyses. Thus, they were not able to determine which opioids or benzodiazepines, contributed to or were actually present at the time of death.
BOTTOM LINE
- For individuals and families seeking recovery: Buprenorphine (an opioid agonist medication best known in formulation with naloxone, marketed as Suboxone) is a medication used for the treatment of opioid use disorder that has been shown to reduce the risk of fatal overdose and support individuals in opioid use disorder recovery. This medication is sometimes prescribed in conjunction with benzodiazepines, a class of sedative medications best known by brand names like Valium and Klonopin). Results from this study support the notion that there may be a significant risk associated with combining these medications. Those taking benzodiazepines in conjunction with buprenorphine were approximately three times more likely to die of overdose, and twice as likely to have an overdose or die of other causes than those taking buprenorphine but not benzodiazepines. At the same time, those combining these medications were also slightly less likely to discontinue buprenorphine treatment, which in and of itself could have protective effects by reducing relapse risk. These findings suggest a high degree of caution should be exercised when considering combining buprenorphine treatment with a benzodiazepine. Safer, non-benzodiazepine ways to manage anxiety and problems sleeping should also be explored, including non-benzodiazepine medications, and non-medication approaches like cognitive behavioral therapy, which can be as effective as medication for managing these issues.
- For treatment professionals and treatment systems: Buprenorphine (an opioid agonist medication best known in formulation with naloxone, marketed as Suboxone) is a medication used for the treatment of opioid use disorder that has been shown to reduce the risk of fatal overdose and support individuals in opioid use disorder recovery. This medication is sometimes prescribed in conjunction with benzodiazepines, a class of sedative medications best known by brand names like Valium and Klonopin). Results from this study support the notion that there may be a significant risk associated with combining these medications. Those taking benzodiazepines in conjunction with buprenorphine were approximately three times more likely to die of overdose, and twice as likely to have an overdose or die of other causes than those taking buprenorphine but not benzodiazepines. At the same time, those combining these medications were also slightly less likely to discontinue buprenorphine treatment, which in and of itself could have protective effects by reducing relapse risk. Based on these findings, caution should be used when considering prescribing a benzodiazepine for an individual taking buprenorphine. Safer, non-benzodiazepine ways to manage anxiety and problems sleeping should also be explored, including non-benzodiazepine medications, and non-medication approaches like cognitive behavioral therapy, which can be as effective as medication for managing these issues.
- For scientists: Buprenorphine (an opioid agonist medication best known in formulation with naloxone, marketed as Suboxone) is a medication used for the treatment of opioid use disorder that has been shown to reduce the risk of fatal overdose and support individuals in opioid use disorder recovery. This medication is sometimes prescribed in conjunction with benzodiazepines, a class of sedative medications best known by brand names like Valium and Klonopin). Results from this study support the notion that there may be a significant risk associated with combining these medications. Those taking benzodiazepines in conjunction with buprenorphine were approximately three times more likely to die of overdose, and twice as likely to have an overdose or die of other causes than those taking buprenorphine but not benzodiazepines. At the same time, those combining these medications were also slightly less likely to discontinue buprenorphine treatment, which in and of itself could have protective effects by reducing relapse risk. More research is needed to better understand the synergistic effects of buprenorphine and benzodiazepines, both in terms of mechanisms of added risk, and mechanisms underpinning any potential benefits. Additionally, more work is needed to explore the potential benefits of combining buprenorphine treatment of opioid use disorder with cognitive-behavioral approaches to manage comorbid psychological disorders such as anxiety disorders.
- For policy makers: Buprenorphine (an opioid agonist medication best known in formulation with naloxone, marketed as Suboxone) is a medication used for the treatment of opioid use disorder that has been shown to reduce the risk of fatal overdose and support individuals in opioid use disorder recovery. This medication is sometimes prescribed in conjunction with benzodiazepines, a class of sedative medications best known by brand names like Valium and Klonopin). Results from this study support the notion that there may be significant risk associated with combining these medications. Those taking benzodiazepines in conjunction with buprenorphine were approximately three times more likely to die of overdose, and twice as likely to have an overdose or die of other causes than those taking buprenorphine but not benzodiazepines. At the same time, those combining these medications were also slightly less likely to discontinue buprenorphine treatment, which in and of itself could have protective effects by reducing relapse risk. Increasing access to buprenorphine treatment will have large public impact in terms of lives saved. Further, risks associated with combining benzodiazepines with buprenorphine potentially could be mitigated by policies and supporting research that give individuals greater access to non-pharmacological ways to manage anxiety and sleep problems including psychotherapeutic approaches such as cognitive behavioral therapy.
CITATIONS
Park, T. W., Larochelle, M. R., Saitz, R., Wang, N., Bernson, D., & Walley, A. Y. (2020). Associations between prescribed benzodiazepines, overdose death and buprenorphine discontinuation among people receiving buprenorphine. Addiction, 115(5), 924-932. doi:10.1111/add.14886

