Patient interest in extended-release buprenorphine for opioid use disorder treatment
Extended-release formulations of buprenorphine that alleviate the burden of daily dosing are available in several countries across North America, Europe, and Australia. Their adoption among the treatment community is partially reliant on patients’ opinions of and willingness to take extended-release formulations. This study assessed the factors associated with having high vs. low interest in extended-release buprenorphine, among French patients receiving opioid agonist treatment (methadone and tablet/sublingual formulations of buprenorphine) for opioid use disorder.
WHAT PROBLEM DOES THIS STUDY ADDRESS?
Buprenorphine (known by the brand name Subutex, or Suboxone when formulated with naloxone) is a medication used for the treatment of opioid use disorder, which helps to ease withdrawal and craving, and prevent opioid overdose. Tablet (taken orally) and sublingual (dissolved under the tongue) formulations are taken as daily doses and have been available for many years. Recently, newer, longer-lasting formulations of buprenorphine (called extended-release buprenorphine), have been approved and are now being prescribed to opioid use disorder patients in various countries (e.g., U.S., France). Administered via implants or monthly injections, extended-release buprenorphine lasts between one week and six months depending on the specific product, and is thought to reduce the burden of daily dosing for patients. It is also shown to produce similar substance use outcomes to tablet and oral formulations. Despite its availability, patients’ acceptance and interest in using these extended-release formulations are also important factors in their adoption within the treatment community.
To gain a better understanding of the facilitators and barriers to extended-release buprenorphine treatment, research has begun to assess patient’s opinions of and willingness to try extended-release buprenorphine. In this study, researchers explored the factors contributing to interest in extended-release buprenorphine among French patients who are receiving other formulations of opioid agonist treatment (i.e., methadone or sublingual/oral buprenorphine).
HOW WAS THIS STUDY CONDUCTED?
This study was a cross-sectional survey (i.e., researchers assess participants at one time point) of 357 adults receiving opioid agonist treatment for opioid use disorder that examined the associations between participant characteristics and interest in extended-release buprenorphine. Survey data were collected between December 2018 and May 2019. Participants were recruited from 68 treatment clinics in France that offered opioid agonist treatment, including 31 outpatient addiction treatment centers, 31 primary care settings, and 6 prison-based medical centers.
Participants responded to survey questions in the following categories: (1) demographics, (2) clinical and opioid use disorder treatment history, (3) patient’s treatment goals, and (4) the pros/cons of daily dosing and agonist treatment. After completing the survey, participants were educated about weekly and monthly treatment with extended-release buprenorphine. Thereafter, participants rated their interest in starting extended-release buprenorphine on a 1-10 scale (later converted to: “low interest” = score of 1 to 6 & “high interest” = score of 7 to 10), and completed survey items about their expectations around treatment with this formulation. The investigators explored the factors associated with high vs. low interest in extended-release buprenorphine, controlling for age and gender.
The large proportion of participants were middle aged (35% aged 35-44 year), unemployed (68%), single (57%) men (74%), with an average of 15 years of opioid use and 10 years of opioid agonist treatment. Sixty-three percent were receiving buprenorphine at the time of the survey, and 38% were receiving methadone.
WHAT DID THIS STUDY FIND?
The researchers used the median score for interest ratings to determine high interest vs. low interest groups, resulting in 55% of patients having high interest in extended-release buprenorphine.
On average, participants rated their interest in extended-release buprenorphine as a 6 out of 10. When patients were categorized into high interest (score of 7-10) vs. low interest (score of 1-6) groups, 196 individuals were designated as having a high degree of interest, whereas 161 had a low degree of interest in starting extended-release buprenorphine.
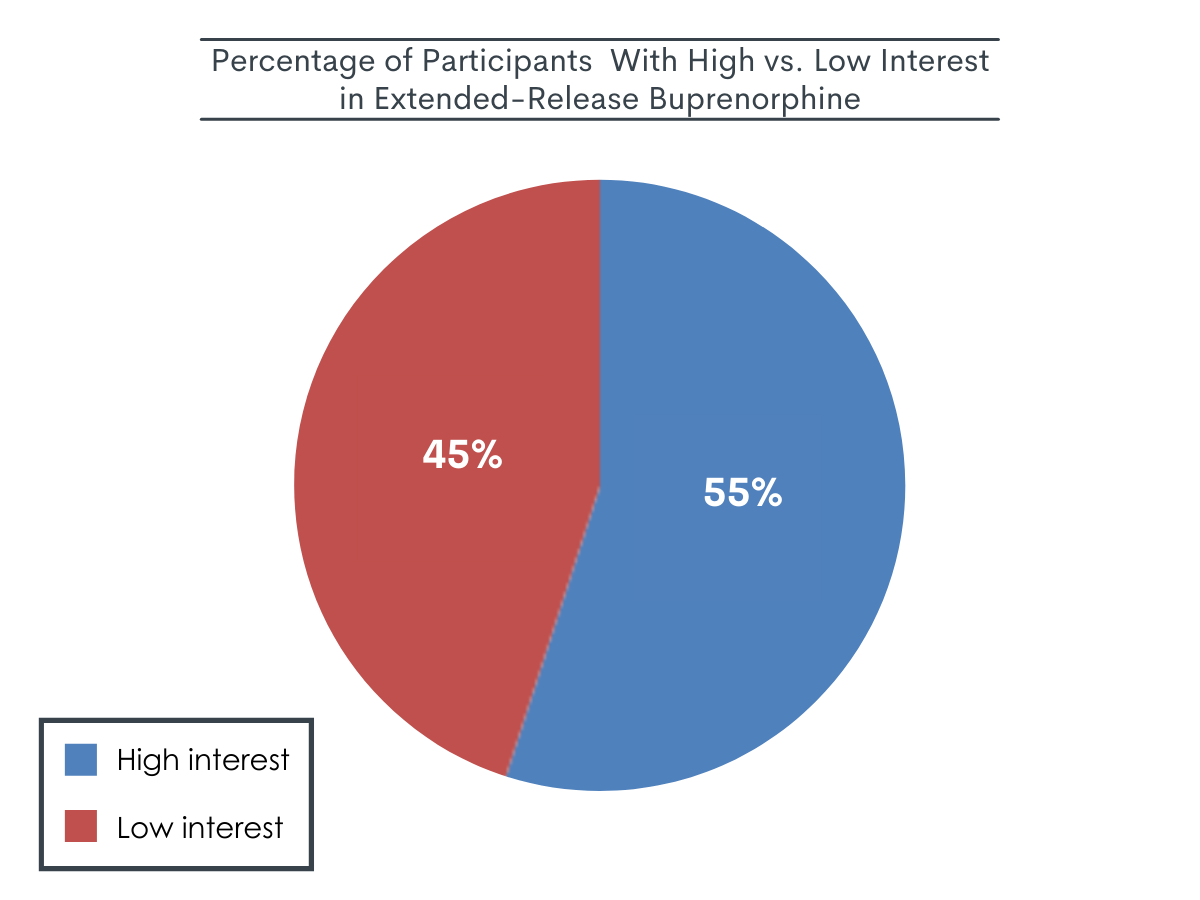
Figure 1. Figure depicts the percentage of patients who were designated as having high interest (Likert scale score of 1-6) or low interest (Likert scale score of 7-10) in starting extended-release buprenorphine.
Lower educational attainment was associated with high interest in extended-release buprenorphine.
Compared to individuals whose highest degree was a high school degree, those with a middle school education (i.e., French secondary school) were 79% more likely to have a high level of interest in extended-release buprenorphine.
The perceived convenience and ease of daily medication dosing was associated with interest in extended-release buprenorphine.
The odds of having a high level of interest in extended-release buprenorphine were 81% greater in patients who frequently forgot to take their current agonist medication and 69% greater among patients who frequently found themselves needing to take it in impractical situations, compared to those who rarely forgot and rarely had to dose in inconvenient situations, respectively. Odds were also greater (118% higher) among individuals who thought daily dosing was constraining, compared to those who did not.
Several factors related to treatment goals and expectations were associated with interest in extended-release buprenorphine.
Individuals whose treatment goals involved stopping the use of illicit drugs were more likely to have high interest in extended-release buprenorphine (67% higher odds than those without this treatment goal), whereas individuals looking to manage their illicit drug use (e.g., reduce but not stop drug use) were more likely to have low interest in extended-release buprenorphine (144% higher odds than those without this treatment goal).
Individuals with expectations that extended-release buprenorphine would reduce craving, health risks, and misuse of their opioid agonist treatment (e.g., injecting, snorting, or smoking their medication), were 138%, 257%, and 88% more likely to have high interest, respectively. The odds of having high interest in extended-release buprenorphine were also higher among those who expected that it would save them money (61% higher odds) and improve social/familial (81% higher odds) or professional (133% higher odds) areas of their life.
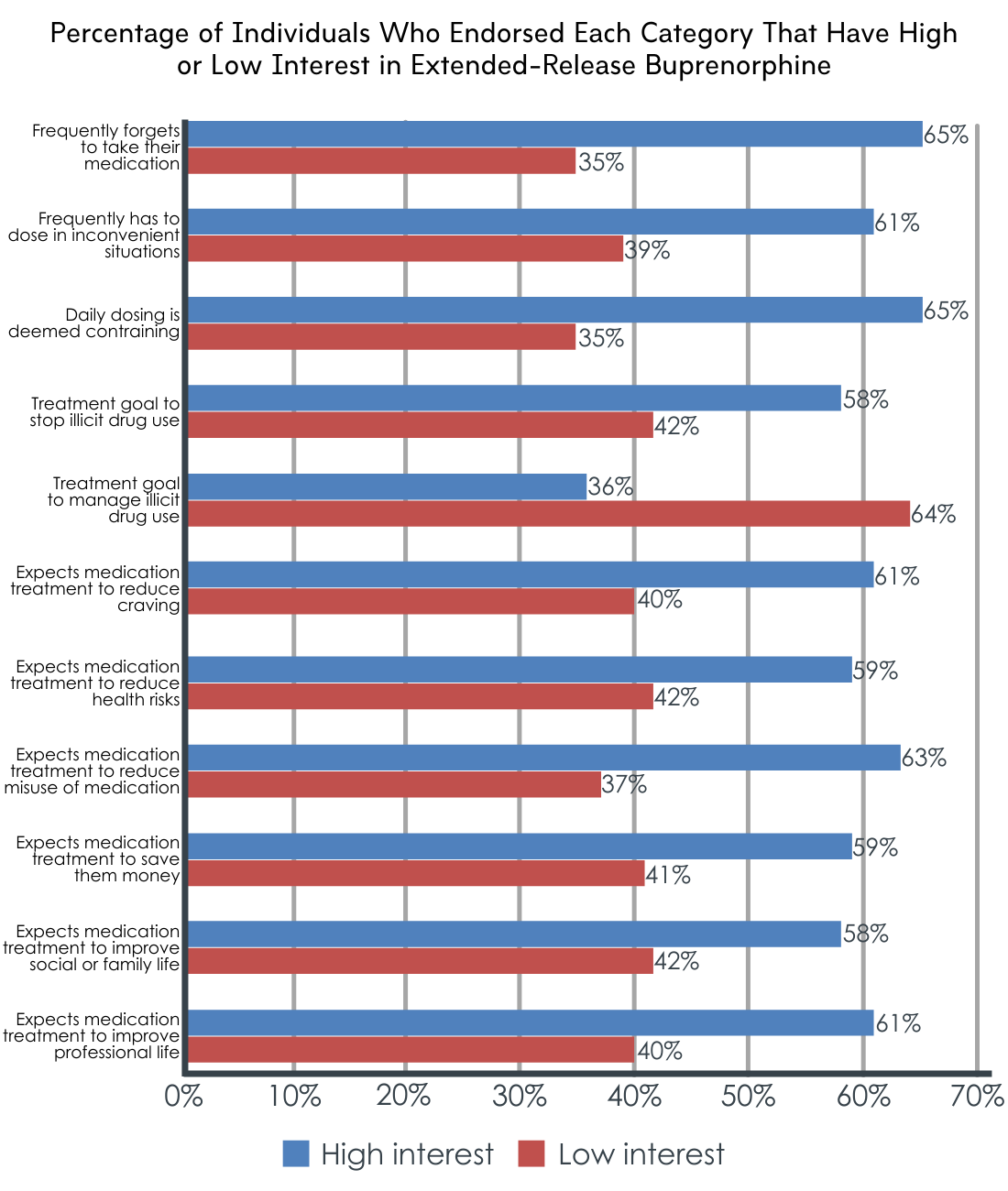
Figure 2. Figure depicts the percentage of individuals who expressed high interest or low interest in extended-release buprenorphine, among those who responded ‘yes’ to a given category (e.g., frequently forgets to take medication).
WHAT ARE THE IMPLICATIONS OF THE STUDY FINDINGS?
Buprenorphine is a much-needed medication for the treatment of opioid use disorder, but daily dosing can be inconvenient. New extended-release formulations have recently become available, which are equally effective as tablet and sublingual formulations of buprenorphine and offer patients more flexibility and freedom with their dosing schedules. However, the adoption of extended-release buprenorphine partially relies on patients’ opinions and willingness to take it. The current study explored factors associated with interest in starting extended-release buprenorphine among opioid use disorder patients undergoing opioid agonist treatment (oral/sublingual buprenorphine or methadone) in France, where extended-release buprenorphine was recently approved for opioid use disorder treatment. The investigators found associations between the degree of interest in extended-release buprenorphine and the following factors: perceived convenience of daily dosing and treatment goals and expectations.
The perceived inconvenience of patients’ current medication regimens (e.g., daily dosing with their current opioid agonist medication) to adherence and daily living appears to play an important role in their level of interest in extended-release buprenorphine. Patients who found daily dosing constraining, frequently forgot to dose, or frequently found themselves having to dose in inconvenient locations were all more likely to be highly interested in extended-release formulations. Although this is not surprising, outcomes highlight the potential utility of extended-release buprenorphine to help patients re-integrate back into the community without feeling enslaved to their medication. Like extended-release formulations, take-home doses that don’t require daily clinic visits can help patients lead normal lives. Studies have revealed several benefits of take-home dosing for patients, including greater convenience, protection of confidentiality, more flexible employment opportunities, feelings of trust, normalcy, and flexibility in daily living activities, as well as less travel and lower costs. Still, concerns around misuse and diversion limit the utility of take-home doses and take-homes do not address issues like forgetting to take one’s medication or having to dose at inconvenient times and locations. With extended-release buprenorphine lasting up to 6 months, it could be an ideal option for those who want more autonomy and flexibility in their treatment and may help retain patients who have difficulty with medication adherence due to forgetfulness.
Regarding treatment goals, extended-release formulations might be more appealing to those who have abstinence-based goals, whereas those who are not willing to cease drug use may be fewer willing candidates. Therefore, extended-release buprenorphine may not be appropriate for patients exclusively seeking harm reduction approaches to treatment and recovery. Furthermore, expectations of the medication’s benefits emerged as one of the most robust predictors of patient interest. Those who expected that extended-release buprenorphine would reduce craving and health risks, and improve social and professional areas of life to support recovery were more likely to be highly interested in it. Therefore, expecting that the medication will do what it is meant to, and support recovery (i.e., both reducing substance use and enhancing other areas of health and well-being), is a straightforward but significant factor in gauging patient interest, and emphasizes the importance of providing patients with evidence-based education around various formulations so that they can make informed decisions. For example, discussing the research findings with patients showing that the tablet/sublingual and extended-release formulations have equivalent efficacy may be key.

Figure 3.
Given the relationship between high interest in extended-release buprenorphine and the belief that it would help patients reduce their misuse of opioid agonist medication, this formulation may also be appropriate for patients who are motivated to achieve recovery but have difficulty with self-restraint and compliance, particularly during the early stages of treatment. Assessing patients’ financial needs when discussing treatment options might also help patients and providers determine the most sustainable treatment option for them, as expectations that extended-release buprenorphine would save patients money predicted greater interest in it.
Although education also demonstrated a statistically significant relationship with interest in extended-release buprenorphine, the magnitude of the effect was small and its interpretation is currently unclear. Further investigation will help determine this finding’s replicability and the factors that might contribute to it. Given limited research in this area (1 study conducted in U.S., 1 in Australia, and 1 in France AKA this study), future research is sure to help us better understand patient preferences and the reasons that motivate these preferences. Together, this information can help us to develop methods that educate patients and enhance their interest in these lifesaving medications to help further address the opioid overdose epidemic.
- LIMITATIONS
-
- All individuals surveyed were already receiving agonist medication (buprenorphine or methadone) and these outcomes might not generalize to the broader population of individuals with opioid use disorder.
- This survey addressed many factors that can potentially influence interest in medication use, but there are likely additional factors that were not assessed here and might influence patient adoption of extended-release buprenorphine, warranting additional research.
- This study was conducted in France and additional research is needed to determine if these findings are replicable in other countries with different cultural and medical perspectives on medication treatment. Furthermore, the French education system differs from the U.S. education system, with U.S. laws requiring school attendance through age 16 and France defining secondary school as a middle school education and primary school as an elementary school education. Thus, outcomes related to education level may not apply to countries with different education systems and regulations.
BOTTOM LINE
Studies like this help us better understand patients’ willingness to utilize available medications for opioid use disorder treatment, and the factors that contribute to it. Focusing on extended-release buprenorphine and a French population of individuals on agonist medications (methadone, oral/sublingual buprenorphine), the research team found that patients who perceived daily dosing as inconvenient and had abstinence-based treatment goals, as well as those who expected that this formulation would improve symptoms and support recovery, were more likely to be highly interested in extended-release buprenorphine. Therefore, extended-release formulations might better support those who feel inconvenienced by daily dosing and offer individuals the freedom and flexibility they need in their daily lives, while supporting their treatment and recovery goals. However, importantly, these formulations are not appealing to all and additional research will help us better understand the patients-level factors that contribute to interest in and outcomes for various medication treatments and their formulations, as well as the mechanisms that underlie these relationships.
- For individuals and families seeking recovery: Extended-release buprenorphine is just as effective as sublingual/tablet formulations of buprenorphine at reducing substance use. If you are seeking abstinence-based recovery and desire a more convenient dosing plan (non-daily dosing), this study suggests you may be more likely to be interested in starting extended-release buprenorphine. Those who are interested are encouraged to speak with their healthcare providers about extended-release buprenorphine to determine if it is right for you.
- For treatment professionals and treatment systems: Given the influence of patient perceptions on the adoption of novel medication treatments for opioid use disorder, it is essential to characterize patient opinions and patient characteristics that might play a role in willingness to utilize these medications. This study demonstrates that individuals with abstinence-based treatment goals, and expectations that the treatment would benefit them, as well as those who find daily dosing with methadone or sublingual/tablet buprenorphine formulations inconvenient or difficult, are more likely to be highly interested in extended-release buprenorphine. As clinicians’ provision of extended-release buprenorphine continues to grow, it will be important to take patient perspectives into consideration when determining the most beneficial treatment plans for individuals. Given the influence of patient expectations on level of interest in medication formulations, it will also be important to educate patients on their individual efficacy and benefits.
- For scientists: Additional research is needed to examine additional predictors not assessed in this study and to characterize the reasons underlying observed relationships between predictors and interest in extended-release buprenorphine. Given that the assessment of treatment goals was a novel component of this study, replication in countries other than France will help determine the generalizability of these findings. It will also be important to determine if patient education around these medications and their effects can help change treatment expectations and, in turn, enhance patients’ interest in utilizing them.
- For policy makers: Extended-release buprenorphine recently became available for the treatment of opioid use disorder and is just as effective as sublingual/tablet formulations of buprenorphine. This study suggests that patients differ in the extent to which they may be interested in extended-release buprenorphine, which has important implications for patient adoption of new formulations. For example, patients who thought extended-release buprenorphine would benefit their treatment and recovery (reduce craving, health risks, etc.) were more likely to be highly interested in it. Therefore, educating the public and treatment communities about the benefits of this formulation and its equivalence to produce positive outcomes when compared with other formulations might ultimately help enhance interest and adoption of it in the community. Additional funding for similar research will advance our understanding of patient perceptions about pharmacotherapy, the factors influencing its adoption and their variability in countries with different healthcare systems and medication attitudes.
CITATIONS
Rolland, B., Trojak, B., Nourredine, M., Bachellier, J., Chappuy, M., Bendimerad, P., . . . Brousse, G. (2021). Determinants of interest in extended-released buprenorphine: A survey among 366 French patients treated with buprenorphine or methadone. Drug and Alcohol Dependence, 220, 108492. doi:10.1016/j.drugalcdep.2020.108492

