Predictors of Continued Use of Extended-release Naltrexone for Opioid Use Disorder
Extended-release naltrexone in combination with psychosocial treatment has been shown to be effective for the treatment of opioid use disorder with rates of abstinence exceeding those of individuals using oral naltrexone or buprenorphine (see here).
WHAT PROBLEM DOES THIS STUDY ADDRESS?
Given the potential benefits of this medication, this study sought to determine what patient factors were associated with treatment adherence among participants in a program sponsored by the Los Angeles (LA) Country Department of Public Health and the University of California, Los Angeles (UCLA) that was designed to increase access to naltrexone.
HOW WAS THIS STUDY CONDUCTED?
This study used data from a program sponsored by LA County and UCLA to increase access to extended-release naltrexone to help prevent alcohol and opioid use. All psychosocial treatment centers funded by LA County were eligible to refer patients to three naltrexone medication hubs.
This study, described in detail here, found that 39 of 431 treatment sites made referrals to the naltrexone medication hubs. In total, 609 patients received naltrexone; this study focused on the 171 patients who received it for opioid use.
Project evaluation consisted of two phases:
- A majority of the participants (n=111) were enrolled during the first phase (April 2010 – December 2011) when extended-release naltrexone was not yet approved for opioid use disorders. Because of this, data on these participants was limited to admission and discharge records.
- In contrast, 60 Phase 2 participants (February 2012 – August 2013) also completed assessments on urges to use opioids. This scale consisted of five questions about cravings, rated from 0 to 6 (6 = highest severity). The ratings were then added to create a cumulative score ranging from 0 to 30.
The study authors (Cousins and colleagues) used data on these participants to address the following questions:
- What patient characteristics are associated with extended-release naltrexone adherence (i.e., receiving two or more medication injections) among individuals with opioid use disorders?
- Do individuals with heroin and non-heroin opioid use disorders differ in extended-release naltrexone adherence?
WHAT DID THIS STUDY FIND?
Of the 171 participants in this study, 54% were male, 66% were non-Hispanic white, and 68% had a heroin use disorder. Heroin users (n=116) reported fewer years of opioid use compared to non-heroin users (n=55). The authors compared this study population (N=171) to all patients in treatment for opioid use disorder in LA Country (N=6101).
Compared to the average patient in treatment for opioid use disorder in LA, participants in this study were more likely to be non-Hispanic White males (36% vs. 22%), be on probation or parole (36% vs. 22%), have a prior mental illness diagnosis (47% vs. 29%), report fewer days of past-month opioid use (10.5 vs. 18 days), fewer days of injection use (5.5 vs. 10 days), and more days of alcohol use (8.5 vs. 6.5 days).
Of the 171 participants receiving naltrexone, the average number of doses received was 2.4.
Buprenorphine “induction” refers to the medically-monitored initiation of treatment and “titration” refers to adjustment of medication to provide a therapeutic effect while minimizing adverse reactions.
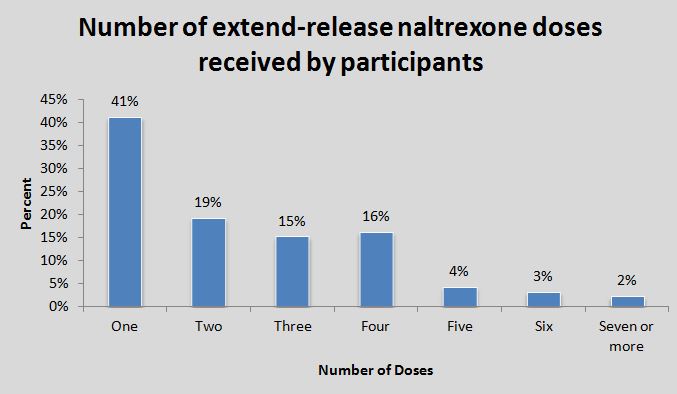
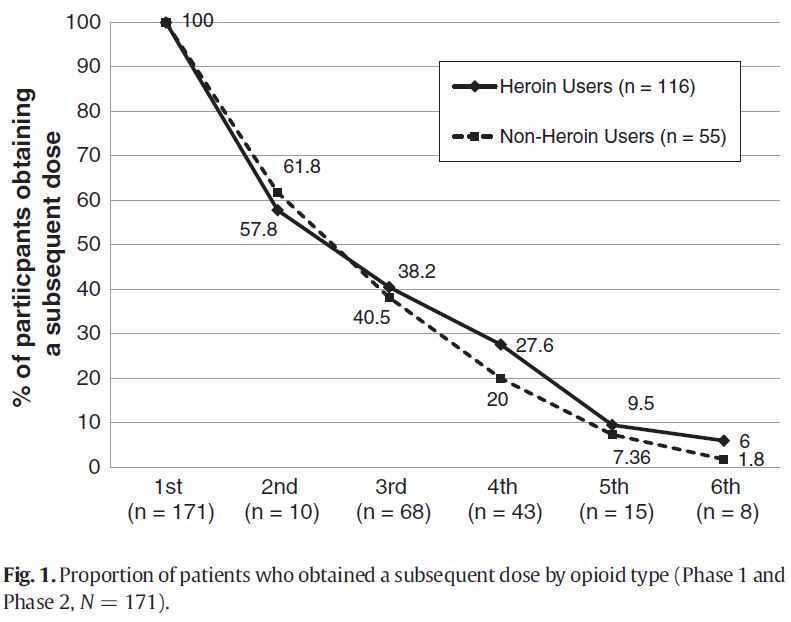
The authors then conducted an analysis to determine what factors were associated with returning for two or more doses (relative to receiving only one).
The results showed that heroin and non-heroin users were equally likely to return for a second dose. After adjusting for patient characteristics (including demographics and drug use characteristics), results showed that individuals who were older and tested for HIV were more likely to receive two or more doses while those who were admitted to the emergency room in the past year and those with a mental health diagnosis were less likely to receive two or more doses.
Additionally, non-heroin users who had injected drugs in the past 12 months were less likely to receive two or more doses.
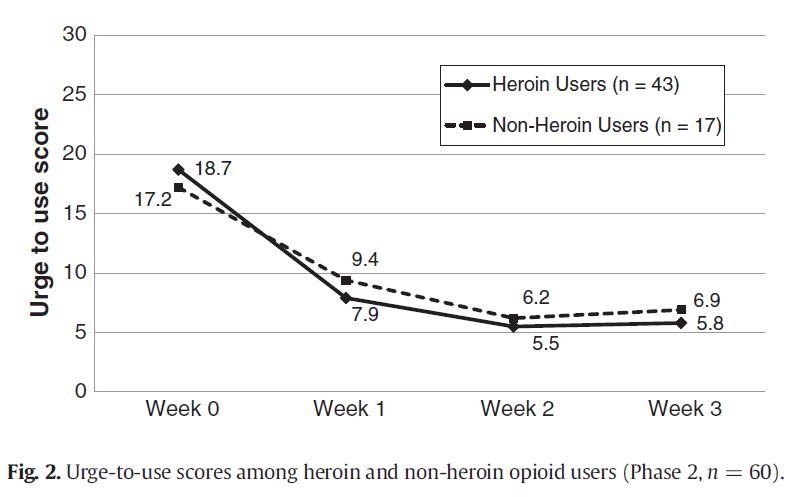
Among phase 2 participants (n=60) completing a weekly survey on urge to use opioids, urge-to-use scores decreased over the first 30 days following initial dose of naltrexone with no differences between heroin users and non-heroin users.
This study found no evidence for differences between individuals with heroin vs. non-heroin use disorder in terms of naltrexone adherence or reduced urge to use opioids following treatment with naltrexone
One distinct group of non-heroin users—those who had injected drugs within the past 12 months—were less likely to receive two or more doses of naltrexone. These individuals may require additional intervention to increase utilization of such programs and obtain the maximum benefits offered by the medication.
WHY IS THIS STUDY IMPORTANT
Since this program was sponsored by and implemented in LA County, it provides an example of how a medication-assisted treatment (MAT) program performs in a real-world setting. Understanding potential predictive associations can help future programs become aware of what types of clients may be more or less likely to adhere to treatment with extended-release naltrexone (i.e., receive more than one dose).
For those who are less likely to adhere to this treatment, more intensive efforts may be needed to help them continue to receive this treatment, in order to maximize potential benefit.
In contrast to previous studies conducted in very specific populations, this study was conducted in a real-world setting with a heterogeneous population, but compared to other studies, found higher rates of treatment dropout and lower average number of naltrexone doses received.
This study suggests that referral to extended-release naltrexone distribution programs from psychosocial treatment is feasible in large, metropolitan settings such as LA County. However, since participants who had received a mental health diagnosis and those who visited the emergency department in the past year were less likely to receive more than one dose of naltrexone, treatment programs may need to increase outreach efforts for such individuals.
- LIMITATIONS
-
- All patients received psychosocial treatment from facilities funded by LA County, but this study did not measure the types of treatments the patients were receiving. Given the breadth of available options, the type and quality of treatment could vary dramatically between participants and ultimately impact the two outcomes of interest in this study: number of additional doses of naltrexone received and urge-to-use scores.
- Also, due to a small sample size, this study may not have had enough statistical power (i.e., the likelihood that a study will detect an effect when there is an effect there to be detected) to find a difference between heroin and non-heroin users in terms of adherence or urge to use.
- Also, actual use of opioids was not assessed as an outcome.
NEXT STEPS
Future studies may wish to incorporate other medication assisted treatment (MAT) options such as methadone and buprenorphine/naloxone (Suboxone) and compare them to extended-release naltrexone in a real-world setting.
Furthermore, studies should examine substance use outcomes over time to determine whether these medications may be safely discontinued for patients who wish to stop taking them.
BOTTOM LINE
- For individuals & families seeking recovery: For opioid use disorder, taking extended-release naltrexone may help decrease urges to use opioids regardless of the type of opioid you use.
- For scientists: This study provides support for extended-release naltrexone’s ability to decrease urge to use opioids with similar results regardless of type of opioid previously used by participants. Future studies should examine substance use outcomes once naltrexone has been discontinued.
- For policy makers: This study used data from a naltrexone distribution program in LA. While results are promising, a cost-effectiveness analysis could determine if these programs save more money (i.e., by reducing opioid use and its consequences) than they cost.
- For treatment professionals and treatment systems: Extended-release naltrexone combined with psychosocial treatment may be a good option for individuals with opioid use disorder. Patients who use heroin or other opioids are likely to experience similar rates of adherence.
CITATIONS
Cousins, S. J., Radfar, S. R., Crevecoeur-MacPhail, D., Ang, A., Darfler, K., & Rawson, R. A. (2015). Predictors of Continued Use of Extended-Released Naltrexone (XR-NTX) for Opioid-Dependence: An Analysis of Heroin and Non-Heroin Opioid Users in Los Angeles County. J Subst Abuse Treat. doi:10.1016/j.jsat.2015.12.004

