Naltrexone Implant Versus Daily Pill
Naltrexone, a medication often prescribed for the treatment of alcohol dependence, can also treat opioid dependence by blocking opioid receptors, helping to prevent relapse when taken daily.
Since a high level of adherence is necessary to reap the benefits of these oral treatment regimens, sustained release formulations of naltrexone could further decrease relapses by eliminating the need for patient adherence to a daily medication regimen. A new abdominal implant containing 1000 mg of naltrexone, approved for use in Russia, has the potential to block the effects of opioids for 2 months.
To test this device, Krupitsky and colleagues completed a 6 month clinical trial in Russia with 306 patients with opioid dependence that had undergone detoxification within two weeks of enrolling in the study. Patients were randomized to three groups described in the table below. All groups received biweekly counseling based on the Individual Drug Counseling model from the Collaborative Cocaine Treatment Study.

There were no significant baseline differences between groups in terms of demographic or clinical characteristics at the start of the study.
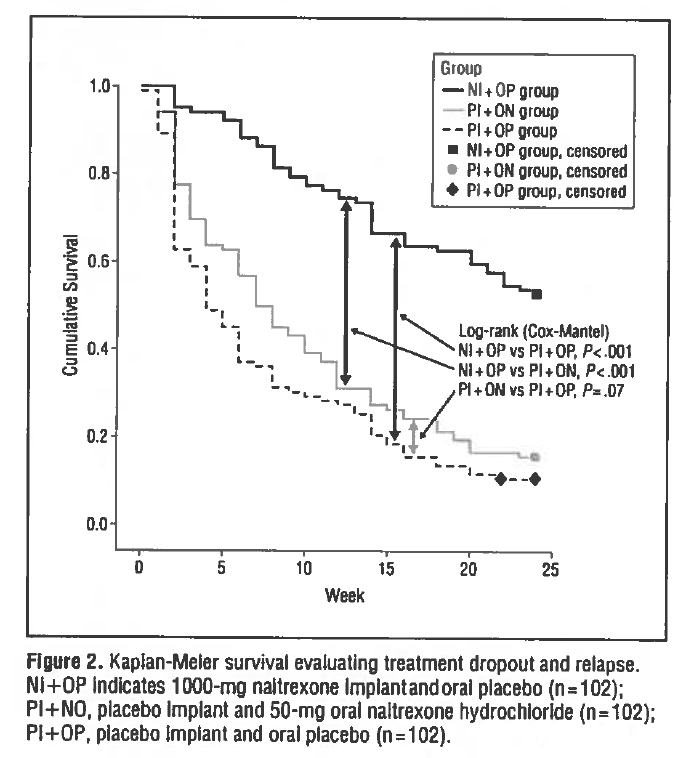
The main objective of this study was to examine the extent to which the three treatment arms retained patients in treatment, defined as not missing two consecutive counseling sessions and not having a relapse. By month six, which marked the end of treatment, the implant group (NI + OP) had a significantly greater proportion of patients who remained in treatment and with no relapse than both the oral naltrexone (PI + ON) and placebo (PI + OP) groups (53% vs. 16% vs. 11%).

IN CONTEXT
Opiate misuse and opiate use disorders have become an alarming public health problem.
Pharmaceutical opiate and heroin overdose deaths between 2000 and 2012 increased from 4,400 and 2,000 to 16,000 and 6,000, respectively. It is critical to develop and test new and innovative approaches to address the problem.
The current study showed that an extended-release naltrexone implant may provide an evidence-based strategy to increase treatment retention and reduce relapse. Importantly, it provides a longer window of protection relative to the month-long injection formulation approved by the US Food and Drug Administration (i.e, Vivitrol).
One of the main values of an extended release formulation is that it takes away the reliance on a patient’s daily motivation to actually take the medication.
Poor outcomes at 12 months for the implant group (7 of 102 with continuous abstinence) compared to 6 months (54 of 102 still in treatment with no relapse) suggests that continued usage is necessary, as six months may not be sufficient time for patients to build the recovery resources needed to maintain abstinence.
- LIMITATIONS
-
Currently, no direct comparison has been made between the injection and the implant forms of naltrexone. Both extended release options have the potential to benefit patients who are naive to this sort of treatment or who have not done well on an oral naltrexone treatment regimen in the past. Maintenance therapy with buprenorphine may yield better long term outcomes (see here) though a direct comparison between the naltrexone implant and buprenorphine is needed.
NEXT STEPS
More research is needed to determine optimal treatment length and/or effective continuing care interventions to help patients maintain gains.
BOTTOM LINE
- For individuals & families seeking recovery: Depending on which versions of naltrexone are approved and available where you live, an extended release formulation of naltrexone, in combination with psychosocial treatment, is likely to improve chances of abstinence from opiates.
- For scientists: The next step appears to be comparisons of the extended release naltrexone implant versus naltrexone injection (e.g.,Vivitrol), the current standard of care.
- For policy makers: Policies that increase opiate users’ access to healthcare providers and systems that can offer extended release naltrexone prescription and administration are likely to reduce overdose deaths, disease and disability, and improve overall public health.
- For treatment professionals and treatment systems: For your opiate using patients, strongly consider referral for extended release naltrexone evaluation if clinically appropriate in combination with other psychosocial interventions and recovery support services.
CITATIONS
Krupitsky, E., Zvartau, E., Blokhina, E., Verbitskaya, E., Wahlgren, V., Tsoy-Podosenin, M., … & Tyurina, A. (2012). Randomized trial of long-acting sustained-release naltrexone implant vs oral naltrexone or placebo for preventing relapse to opioid dependence. Archives of general psychiatry, 69(9), 973-981.

