Buprenorphine (Subutex) is safe for women during pregnancy – but does medication matter for the health of their newborn babies?
Pregnant women with opioid use disorder are typically maintained on opioid medication through pregnancy, and their babies medically tapered from opioids immediately following birth. Buprenorphine (which is frequently known by its common brand name, Subutex) is a widely used opioid medication considered safe for women during pregnancy. However, little is known about how the dose of buprenorphine prescribed might affect newborn babies. This study explored if higher doses of buprenorphine during pregnancy lead to greater severity of neonatal abstinence syndrome.
WHAT PROBLEM DOES THIS STUDY ADDRESS?
Medical guidelines indicate that pregnant women with opioid use disorder should be maintained on opioid agonist medications, like buprenorphine or methadone, during their pregnancy, as opioid withdrawal can cause significant medical complications to mother and child during gestation. Although outcomes for both mother and baby are improved by this approach, it means babies can be born with physiological dependence to opioids. If medication is not provided for such newborns, then they may experience neonatal abstinence syndrome, characterized by hyper-irritability of the central nervous system, gastrointestinal tract, and respiratory tract. In other words, infants born with physiological dependence to opioids may be given opioid medications (usually morphine) soon after birth to reduce withdrawal symptoms and are gently tapered off over 1-2 weeks. Exposure to these medications to treat neonatal abstinence syndrome is not thought to have any long-term adverse health effects for infants.
Previous research has shown that infants exposed to buprenorphine require less and shorter treatment courses with morphine to treat neonatal abstinence syndrome compared with infants exposed to methadone during pregnancy. The authors explored whether the dose size of buprenorphine taken by mothers is related to severity of infants’ neonatal abstinence syndrome.
HOW WAS THIS STUDY CONDUCTED?
The authors conducted a retrospective analysis of mother–infant dyads treated with buprenorphine from 2000 to 2016 in an urban, Washington State hospital using medical record review. Mother–infant dyads were included in the analysis if mothers were 18 to 53 years of age, diagnosed with Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) opioid dependence (consistent with opioid use disorder moderate or severe in the DSM-5) and being treated with buprenorphine.
Exclusion criteria included opioid use relapse leading to transition to methadone maintenance during pregnancy, no drug screen being performed at the time of delivery, and drug screen positive for opiates, amphetamines, methamphetamines, cocaine, benzodiazepines, MDMA, PCP, oxycodone, and/or methadone, or negative for buprenorphine. Individuals who tested positive for marijuana were included.
The authors explored whether mothers’ buprenorphine dose at the time of delivery was associated with whether babies required treatment with morphine for neonatal abstinence syndrome (yes/no). They also explored among babies who did experience neonatal abstinence syndrome, whether buprenorphine dose was related to: 1) neonatal abstinence syndrome severity (assessed using the Finnegan Neonatal Abstinence Scoring Tool, which scores 21 signs of withdrawal), 2) peak morphine dose used to manage infants’ withdrawal symptoms, 3) time to morphine initiation, 4) days on morphine, and 5) total duration of infants’ hospital stay.
WHAT DID THIS STUDY FIND?
A total of 89 women and their babies met all eligibility criteria for study inclusion. Incidence of neonatal abstinence syndrome requiring morphine medication overall was 43.8% (39 out of 89 infants). Treated infants averaged 16 days on morphine with a total hospital stay of 20 days, whereas infants who did not require medication for neonatal abstinence syndrome had an average stay of 5 days.
Buprenorphine dose was not associated with neonatal abstinence syndrome severity.
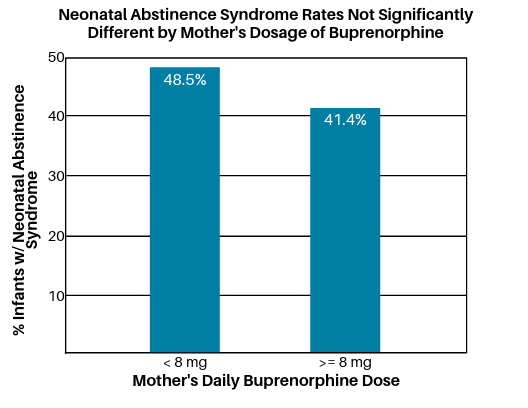
When women were grouped based on their dose of buprenorphine, 33 women had buprenorphine doses of 8 mg per day or less, and 56 women had buprenorphine doses above 8 mg per day. The incidence of neonatal abstinence syndrome requiring medication, however, was not statistically significantly different between these two groups (48.5% for mothers taking <8mg, versus 41.4% for mothers taking >8mg). In other words, the authors did not find statistical evidence suggesting between-groups differences on this measure were greater than would be expected by chance.
Among infants requiring morphine medication (n= 39), mothers’ buprenorphine dose was not associated with neonatal abstinence syndrome severity, peak morphine dose used, time to morphine initiation, days on morphine, and total duration of infants’ hospital stay, suggesting buprenorphine dose does not affect these measures. The possible exception may be number of days infants required morphine, which showed a statistical trend suggesting infants whose mothers were prescribed higher doses of buprenorphine required longer morphine tapers. This is perhaps not surprising since these infants would be physiologically used to higher doses of opioids.
Breastfeeding appears to help with neonatal abstinence syndrome.
Notably, the authors also found that infants who were exclusively breastfed were statistically significantly less likely to require morphine medication. Further exploration of this revealed that infants who were exclusively breastfed also had mothers who attended more prenatal visits, smoked fewer cigarettes daily, and had less SSRI anti-depressant medication use, suggesting a combination of these factors most likely explained why these infants were less likely to have neonatal abstinence syndrome and require treatment with opioid medication. Additionally, it is known that buprenorphine is transferred to the infant through breast milk, such that breastfed babies are receiving small doses of the medication that reduces withdrawal symptom, which likely explains some of the observed effect.
WHAT ARE THE IMPLICATIONS OF THE STUDY FINDINGS?
Overall, the authors’ findings suggest there is no association between maternal buprenorphine dose during pregnancy and incidence and severity of neonatal abstinence syndrome. This finding—if replicated in a larger sample—is important because previous research has shown that buprenorphine doses of 16+ mg are associated with reduced opioid use, and thus may help pregnant women with opioid use disorder avoid opioid use during pregnancy. Given this favorable risk-benefit ratio, providers may feel comfortable going to higher doses of buprenorphine if needed to keep women engaged in care, minimize cravings/withdrawal, and reduce risk of relapse.
Findings also support present literature encouraging breastfeeding for infants with neonatal abstinence syndrome. Particularly in the setting of America’s current epidemic of opioid use, where the rate of opioid use during pregnancy is approximately 5.6 per 1000 live births, these data provide more support for the safety of buprenorphine treatment of pregnant women with opioid use disorder. At the same time, these findings should be viewed in light of the fact that the authors excluded individuals who had tested positive for drugs other than opioids and cannabis, thus potentially excluding individuals with more severe substance use disorder from the study. It’s therefore possible the study findings don’t generalize to all babies born to women taking buprenorphine. Additionally, it is not clear if the authors considered premature births in their study, which could have affected results in unknown ways since premature babies commonly experience certain symptoms also observed in neonatal abstinence syndrome.
- LIMITATIONS
-
- The study’s sample size was small, thus limiting the authors’ ability to statistically detect associations between buprenorphine dose and infant neonatal abstinence syndrome symptoms. It is possible that with a larger sample size, observed correlations between buprenorphine dose and neonatal abstinence syndrome may be statistically significant. As such, the findings should be interpreted with caution.
- Participants who relapsed and needed to be transitioned to methadone, and those who tested positive for drugs other than cannabis, were excluded from the study. Thus, the findings of this research may not generalize to those with more severe opioid use disorder.
- Prenatal care has changed some over the 16-year period of the study, and this may have some effect on results.
- Nine infants requiring morphine for neonatal abstinence syndrome were started on morphine doses lower than the normal 0.05 mg/kg due to provider concern for other medical issues or infant sedation. These infants, when compared with the other infants who received protocol-based initial doses of morphine, had no difference in sex, maternal age, maternal buprenorphine dose, time to start morphine, or peak neonatal abstinence syndrome scores, but did have fewer prenatal visits (mean visits 4 for lower-dose morphine vs 7.4), and lower peak morphine doses. It may be these nine infants represent a subset of infants with complex presentations due to medical issues.
- Two infants treated for neonatal abstinence syndrome also received the sedative clonidine to help symptoms. The authors note that this may have affected peak morphine dose and days of treatment.
BOTTOM LINE
- For individuals and families seeking recovery: Maintaining women on opioid use disorder medications during pregnancy is now the standard practice in medicine, as it reduces risk to mother and baby. Pregnant women or women thinking about becoming pregnant who have opioid use disorder should consult with their obstetrician/gynecologist, but based on these authors’ preliminary findings, there appears to be no substantial added benefit of reducing buprenorphine dose during pregnancy, though larger-scale studies on this issue are needed before clear guidelines can be established. Also, if possible, exclusively breastfeeding appears to benefit infants born with neonatal abstinence syndrome.
- For treatment professionals and treatment systems: Findings suggest there is no substantial added benefit of reducing buprenorphine dose during pregnancy in terms of likelihood and severity of neonatal abstinence syndrome, though larger-scale studies on this issue are needed before clear guidelines can be established. Where possible, mothers should be encouraged to exclusively breastfeed, as this may ameliorate neonatal abstinence syndrome.
- For scientists: Findings suggest there is no substantial added benefit of reducing buprenorphine dose during pregnancy in terms of likelihood and severity of neonatal abstinence syndrome, though larger-scale studies on this issue are needed before clear guidelines can be established. Future studies on whether partial breastfeeding confers beneficial results similar to exclusive breastfeeding are warranted.
- For policy makers: Findings suggest there is no substantial added benefit of reducing buprenorphine dose during pregnancy in terms of likelihood and severity of neonatal abstinence syndrome, though funding for larger-scale studies on this issue should be provided in order for clearer guidelines to be established. Improving access to medical care, as well as medications like buprenorphine for women with opioid use disorder, is paramount to ensure optimal outcomes for mothers and their infants.
CITATIONS
Wong, J., Saver, B., Scanlan, J. M., Giantusos, L. P., Bhakta, Y., Walsh, J., . . . Rudolf, V. (2018). Does maternal buprenorphine dose affect severity or incidence of neonatal abstinence syndrome? Journal of Addiction Medicine, 12(6), 435-441. doi: 10.1097/ADM.0000000000000427

