Injectable buprenorphine for opioid use disorder: Effects on 1-year recovery outcomes
An extended release version of buprenorphine was recently approved by the FDA to treat opioid use disorder. Prior research has revealed the short-term benefits of buprenorphine on traditional treatment outcomes like substance use and acute harm (e.g., overdose). Building upon this literature, the current study assessed the longer-term effects of monthly buprenorphine injections on patient-centered outcomes like quality of life and health status, suggesting improved well-being up to 1 year of treatment.
WHAT PROBLEM DOES THIS STUDY ADDRESS?
Medications for opioid use disorder are effective lifesaving therapies that promote successful treatment and enhance recovery outcomes. Opioid agonist medications, like buprenorphine, provide stable low-level stimulation of opioid receptors and block the effects of other opioids (e.g., heroin). The FDA recently approved an extended release version of buprenorphine, which is administered via monthly injection for the treatment of opioid use disorder. Previous studies have demonstrated the ability of monthly buprenorphine injections to enhance recovery outcomes after six months of treatment. However, little is known about its longer-term effects. Also, for studies on opioid use disorder medications generally, outcomes of health and well-being beyond substance use and acute harms like overdose are particularly lacking. With more scientific knowledge on health and well-being outcomes among those who are prescribed opioid use disorder medications, stakeholders can determine whether other services may be needed to help improve these quality of life outcomes. To address this gap, the current study examined the effects of monthly buprenorphine injections on patient-centered outcomes after up to 12 months of treatment.
HOW WAS THIS STUDY CONDUCTED?
The authors conducted a single-group longitudinal investigation of the effects of monthly buprenorphine injections in opioid use disorder patients. Data were collected as part of an open-label safety study of extended-release buprenorphine injections for opioid use disorder treatment that did not include a control group such as placebo medication (open-label study means that both the researchers and the participant know which treatment the participant is receiving and in this case there was no control group so everyone of course knew patients were getting the monthly buprenorphine injections). Participants completed the following procedures in the order presented: (1) seven-days of screening to assess participant eligibility; (2) three to 14 days of sublingual buprenorphine treatment (i.e., a stabilization period to ensure patients wouldn’t experience adverse reactions to the active ingredient in monthly buprenorphine injections); (3) 45 weeks of extended-release buprenorphine injections (initial injection of 300 mg, with subsequent injections of 100 mg or 300 mg according to the clinician’s judgement), and (4) follow-up assessment four weeks after the last buprenorphine injection (i.e., study week 49).
Patient-centered measures were collected at three time points: screening, baseline (i.e., after the 3-14-day stabilization period, and before the first injection), and again at follow-up (i.e., study week 49; after ~12 months of treatment). Measures of interest included: (1) health status measured along 5 dimensions of health (mobility, self-care, daily activities, pain/discomfort, and anxiety/depression; i.e., EQ-5D-5L inventory); (2) quality of life as it relates to mental and physical health (i.e., SF-36v2 inventory); (3) patient perceptions of treatment effectiveness in various domains (substance use, health (e.g., physical/emotional health), lifestyle (e.g., housing, employment, interpersonal relationships), and community involvement (e.g., being a responsible and contributing member of society); i.e., Treatment Effectiveness Assessment); (4) severity of addiction-related problems, including employment status, alcohol and drug use, as well as medical, legal, family/social, and psychiatric problems; (5) patient satisfaction with medication treatment (yes/no; derived from the Medication Satisfaction Questionnaire).
Four hundred and twelve participants initiated injectable buprenorphine treatment. Participants were predominantly white (72%) men (64%), with a diagnosis of moderate or severe opioid use disorder (based on the diagnostic and statistical manual of mental disorders, 5th edition, or DSM-5) and an average age of 38 years old. The majority of participants (81%) consistently received the 300 mg dose of injectable buprenorphine throughout the study. Dose reductions from 300 mg to 100mg occurred in 19% of participants (11 of the 80 participants with dose reductions eventually increased their dose back to 300 mg). Consistent with other opioid use disorder medication trials, 50% of participants discontinued their treatment by the end of the study (i.e., final follow-up completed with 204 of the 410 individuals who completed baseline). All individuals were included in the analysis, but follow-up data was not available for all participants at all time points. Given that 50% dropped out before final follow-up assessment (at 12 months), outcomes should be interpreted with caution and understanding that this may be a less severe and higher functioning sub-population.
WHAT DID THIS STUDY FIND?
Overall health status improved from screening to baseline.
Health status improvements occurred relatively early on in study participation. Improvements were observed at baseline, when participants had completed the stabilization period (i.e., sublingual buprenorphine treatment in preparation for monthly buprenorphine injections) but had not yet started extended-release injectable buprenorphine. Health status remained stable thereafter, with follow-up measures among those who completed the study at month 12 being similar to those collected at baseline.
Health-related quality of life improved from screening to baseline and from baseline to follow-up.
Both physical and mental health-related quality of life improved after the stabilization period. Mental health-related quality of life continued to show improvements among those who completed the study after 12 months of treatment.
Severity of addiction-related problems decreased from baseline to follow-up.
Medical problems, employment status, drug use, legal status, family/social status, and psychiatric problems all improved after ~12 months of injectable buprenorphine treatment.
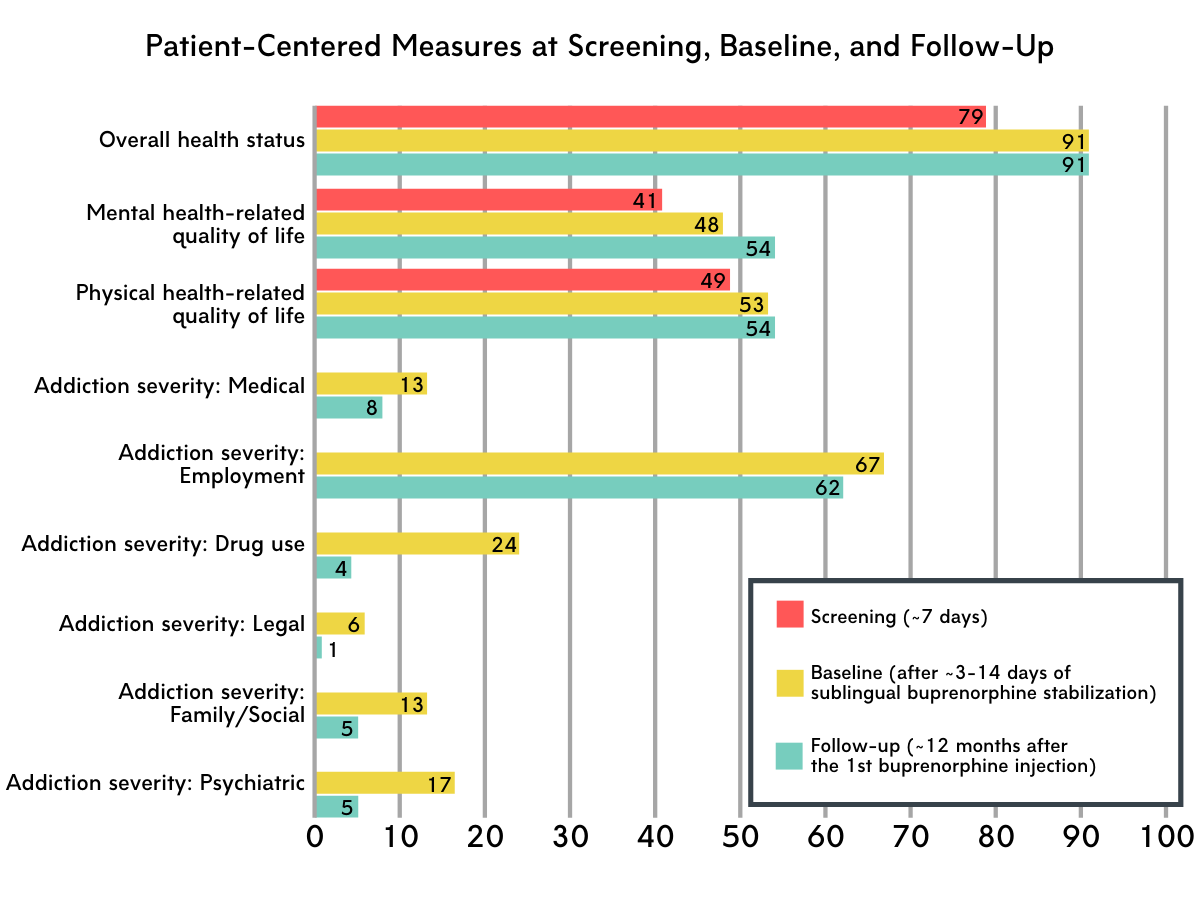
Figure 1 depicts patient-centered outcomes over the course of the study. Scores for all measures range from 0-100. Note: Higher addiction severity scores indicate more severe problems and, potentially, lower functioning. For all other measures, higher scores indicate better functioning. For mental and physical health-related quality of life, a score of 50 represents the U.S. population norm.
Perceived treatment effectiveness increased over the course of the study.
The perceived effectiveness of treatment increased from screening to baseline and from baseline to follow-up (12-months) for those who remained in the study. Enhanced effectiveness was noted in all domains assessed, including effectiveness at reducing substance use and benefiting health, lifestyle, and community involvement.
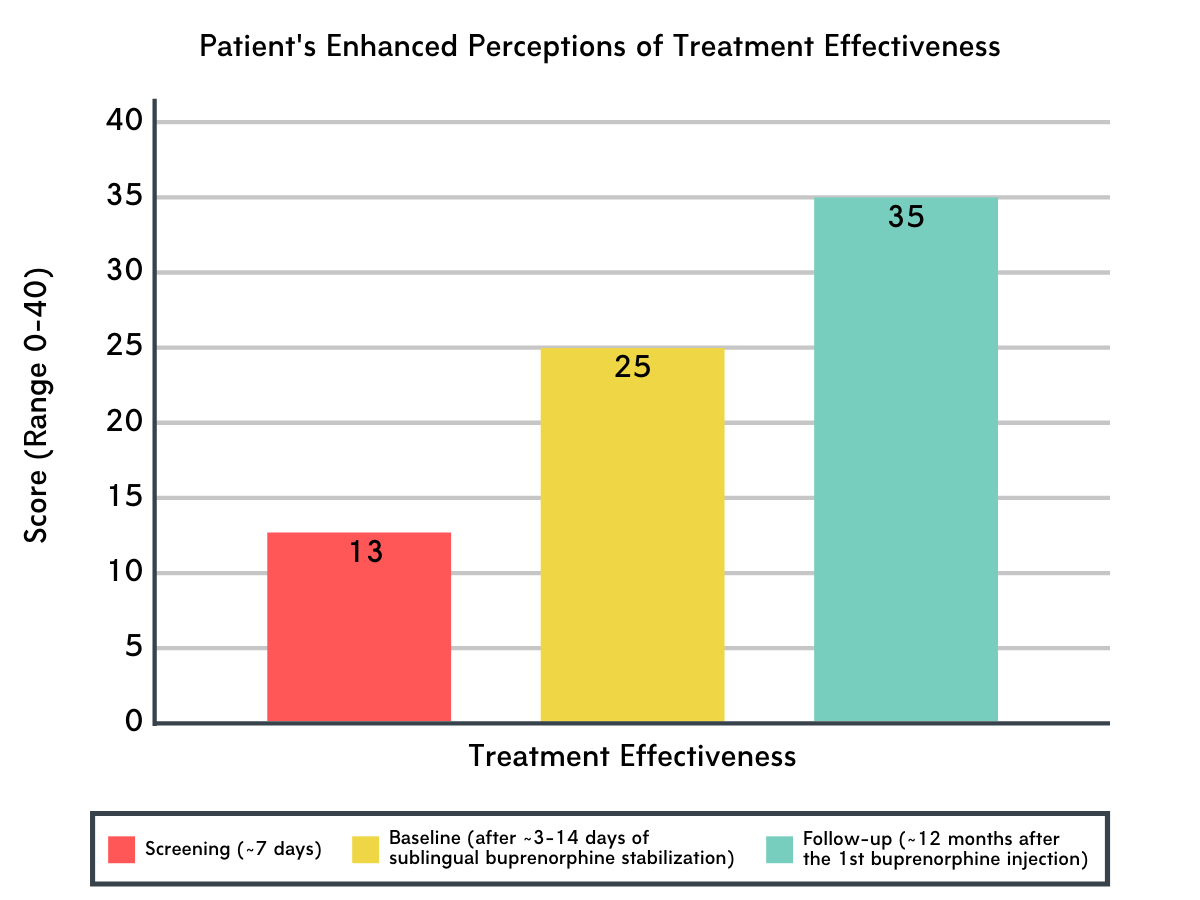
Figure 2.
Relatively high rates of medication satisfaction were reported throughout the study’s duration.
Medication satisfaction remained relatively stable throughout the study, with 85% and 89% of participants reporting that they were satisfied with their injectable buprenorphine treatment at week 9 and week 49 (follow-up), respectively.
WHAT ARE THE IMPLICATIONS OF THE STUDY FINDINGS?
In prior studies, monthly extended-release buprenorphine injections have shown to be equally as effective as daily buprenorphine administration via sublingual/tablet formulations, but its longer-term patient-centered outcomes have yet to be fully characterized, as previous studies have focused on substance use (e.g., % days of substance use) and abstinence-related outcomes only. Recovery is multidimensional and experts suggest its measurement should go beyond substance use alone. Thus, capturing patient-centered outcomes like quality of life and well-being is of increasing importance.
This study included less common outcome measures in addiction treatment research, and thus helps advance a more comprehensive understanding of the longer term (12 month) effects of injectable buprenorphine. Over the course of the study, patients showed improvements in health status, health-related quality of life, treatment effectiveness, and addiction severity. Although many improvements occurred before the onset of monthly buprenorphine injections, and were evident following receipt of sublingual buprenorphine during the stabilization phase of the treatment protocol, very importantly these benefits were maintained after transitioning to monthly buprenorphine injections, highlighting the benefits of the active ingredient in these medications (i.e., buprenorphine) regardless of the specific formulation. Some measures continued to improve as far out as 12 months after starting treatment, including treatment effectiveness, quality of life as it pertains to mental health, and addiction severity.
Notably, almost no declines were seen in the outcome measures of interest. Although 12-month outcome measures were only available for study completers, possibly reflecting a less severe/better prognosis sub-population of patients, results suggest the potential of monthly buprenorphine injections for maintaining benefits through 12-months, and support previous evidence of buprenorphine’s ability to improve outcomes. For example, a prior randomized controlled trial demonstrated buprenorphine’s ability to improve substance use outcomes and employment rates after six months of treatment, above and beyond individual drug counseling. Given that only 51% of individuals in the current study were employed at the 12-month follow-up, and that the average age of participants was relatively young (38 years), additional services beyond medication treatment may be needed to help individuals obtain and maintain employment long term.
Providing social support may also help to bolster improvements in interpersonal functioning and community involvement. It is worth noting that physical health-related quality of life did not improve beyond baseline and this lack of improvement may be due to a subtle but specific decline in ‘physical functioning’ among participants (one of the domains used to assess overall physical health-related quality of life). Although mental and physical health-related quality of life scores at the 12-month follow-up were equivalent to U.S. population norms, additional support may be needed for management of new and/or chronic physical health problems to ultimately reduce the impact of physical health ailments on daily functioning and promote successful recovery.
Importantly, those participants who stayed on the medication treatment and continued to participate in the study reported a high level of satisfaction with their medication treatment; only about 10-15% of patients reported treatment dissatisfaction between week nine and 49 of treatment, highlighting patient receptivity of injectable buprenorphine treatment at least for a large proportion of those who received it and stuck with it. Pharmacotherapy discontinuation remains an ongoing issue in the broader opioid use disorder treatment population, and studies that develop and test ways to enhance treatment retention could improve medication outcomes and reduce the overall public health burden of opioid use disorder.
- LIMITATIONS
-
- Individuals with co-occurring moderate/severe substance use disorders, such as alcohol use disorder, were excluded from this investigation. Given the relatively high prevalence of co-occurring substance use problems in opioid use disorder populations (~66%), additional research is needed to determine if these outcomes also generalize to individuals with more severe addiction histories. Given that the majority of participants were white men, research is also needed to evaluate the translation of these findings in other patient populations.
- 2. Of the 412 participants that initiated injectable buprenorphine in the study, 50% discontinued treatment at some point during the study, with the most common reason being that the participant was lost to follow up or withdrew their consent. Those remaining in the study may therefore represent a higher functioning and less severe sub-population of patients. Although the authors found consistent results in sensitivity analyses conducted among those who completed the treatment only (as opposed to outcomes presented above, which included everyone regardless of missing data points), those who did not complete the 12-month follow-up were not included in analyses looking at change from baseline to the end of the study. Therefore, further investigation is needed to better understand the reasons underlying treatment drop out and to assess the external validity of the findings reported here.
- This study only assessed extended-release injectable buprenorphine treatment. Additional research is needed to determine how other opioid use disorder medications like naltrexone, methadone, and sublingual/tablet formulations of buprenorphine affect similar quality of life outcomes.
- Given the lack of a control or comparison treatment group, it is impossible to know the degree to which similar patients would fare on these outcomes without injectable buprenorphine and, thus, the extent to which any improvements can be attributed directly to the medication rather than other interventions or the passage of time is unknown.
BOTTOM LINE
- For individuals and families seeking recovery: This longitudinal study evaluated the effects of extended release buprenorphine, administered as a once-monthly injection, on 12-month outcomes relevant to health and well-being. For the roughly half of patients who did not drop out of the study, they showed improved health status (e.g., mobility, self-care, ability to carry out daily activities), health-related quality of life (e.g., mental health, vitality, social functioning), addiction severity (e.g., fewer problems related to substance use and psychiatric status), and perceptions of treatment effectiveness. Many of these improvements occurred during the stabilization phase of treatment (when participants received sublingual buprenorphine prior to monthly buprenorphine injections) and were either maintained or continued to improve as far out as 12 months after the first injection. Previous studies have shown that injectable buprenorphine is equally effective as daily buprenorphine administration via sublingual/tablet formulations at enhancing traditional treatment outcomes, like abstinence, after ~ 6 months of treatment. This study included less common outcome measures in addiction treatment research, and thus helps advance a more comprehensive understanding of the longer term (12 month) effects of injectable buprenorphine. It suggests the ability of injectable buprenorphine to maintain and promote improvements in well-being upwards of 12 months after the first dose. Despite observed improvements, results further suggest the ongoing need for support services that complement medication to help facilitate these gains (e.g., support services that further enhance physical health-related quality of life, and provide linkage to employment opportunities). Individuals interested in injectable buprenorphine treatment are encouraged to contact their health care and insurance providers to inquire about whether it is right for them.
- For treatment professionals and treatment systems: Extended release buprenorphine injections are shown to be equally effective as other buprenorphine formulations (once daily sublingual/tablet formulations) at treating opioid use disorder and enhancing traditional treatment outcomes (e.g., abstinence), but its longer term effect on less traditional outcomes like health and well-being are largely unknown. This longitudinal study evaluated the effects of injectable buprenorphine on 12-month health and well-being outcomes among individuals with opioid use disorder. Over the course of the study, patients showed improved health status, health-related quality of life, and addiction severity, and enhanced perceptions of the treatment’s effectiveness. These benefits occurred early on in treatment, after a brief period of stabilization with sublingual buprenorphine and prior to monthly buprenorphine injections. For those who responded to the end of treatment measures at 12 months (~50% of the original patient sample), these benefits were maintained, and some measures continued to improve after the first injection, including treatment effectiveness, quality of life as it pertains to mental health, and addiction severity. Assessing outcomes like quality of life and well-being are essential for ensuring optimal treatment delivery and promoting long-term recovery. This study’s results support previous evidence of buprenorphine’s ability to improve treatment outcomes, and suggest injectable buprenorphine’s ability to help maintain improvements in health and well-being in the long term.
- For scientists: Extended release injectable buprenorphine is shown to be equally effective as other buprenorphine formulations (once daily sublingual/tablet) at treating opioid use disorder and enhancing traditional treatment outcomes (e.g., abstinence). However, its long-term effects have yet to be fully characterized and studies have largely focused on traditional treatment outcomes (e.g., abstinence; % days substance use). Evaluation of patient-centered outcomes that address health and well-being is needed to gain a more comprehensive understanding of opioid use disorder treatment and recovery. The current study examined the effects of injectable buprenorphine on 12-month patient-centered outcomes. Patients reported improvements in health status, health-related quality of life, addiction severity, and treatment effectiveness. These benefits initially occurred after a brief introduction to buprenorphine via a sublingual buprenorphine stabilization period, complementing previous reports of buprenorphine’s proximal benefits. Among those who completed 12-month end-point measures, these benefits were maintained or continued to improve after up to 12 months of injectable buprenorphine treatment, highlighting the potential of monthly buprenorphine injections to support treatment and recovery long term. Importantly, analysis of 12-month outcomes only included those who had completed the end-of-treatment measures, suggesting these individuals may be a less severe/better prognosis subset of those who started the open-label study severely limiting conclusions. Therefore, research is needed to determine whether these outcomes translate to more severe opioid use disorder populations, and to identify the optimal treatment duration for supporting patient outcomes. Incorporating measures of health and well-being into future addiction research will ultimately advance a more comprehensive understanding of treatment and recovery.
- For policy makers: The FDA recently approved an extended release version of buprenorphine for the treatment of opioid use disorder. Previous studies have demonstrated its effectiveness and positive outcomes at six months of treatment, but little is known about the long-term effects of injectable buprenorphine, particularly as they pertain to outcomes like quality of life and daily productivity which are essential for ensuring optimal treatment delivery and promoting long-term recovery. This longitudinal study addressed this gap by evaluating the effects of injectable buprenorphine on 12-month outcomes relevant to health and well-being, and demonstrated improvements in all measures, including health status, health-related quality of life, addiction severity, and treatment effectiveness. These benefits initially occurred after patients took sublingual buprenorphine during a stabilization phase, and were maintained or further improved among study completers, after up to 12 months of treatment with injectable buprenorphine. Therefore, this study complements previous evidence of buprenorphine’s effectiveness and furthers our understanding of less traditional addiction treatment outcomes by suggesting injectable buprenorphine may help maintain and further improve multiple facets of well-being long term. Additional funding to better characterize health, quality of life, and similar outcomes in opioid use disorder patients and the role that various pharmacotherapies play would ultimately help address the opioid epidemic by lending insight to the factors that require additional attention during clinical treatment and recovery support.
CITATIONS
Ling, W., Nadipelli, V. R., Solem, C. T., Ronquest, N. A., Yeh, Y. C., Learned, S. M., . . . Heidbreder, C. (2020). Effects of monthly buprenorphine extended-release injections on patient-centered outcomes: A long-term study. Journal of Substance Abuse Treatment, 110, 1-8. doi: 10.1016/j.jsat.2019.11.004

