Does Injectable Vivitrol Increase or Decrease Medical Healthcare Utilization Among Opioid Dependent Patients?
Injectable Naltrexone (brand name Vivitrol) has emerged as an evidence-based medication for opioid use disorder that has appealing qualities including no daily dosing requirements, no agonist properties that can induce drowsiness in some, and potentially reduced stigma. This study sought to determine the effect of medication on other forms of hospital-based healthcare utilization during the first year and a half.
WHAT PROBLEM DOES THIS STUDY ADDRESS?
Opioid use disorders and related consequences like drug overdose have continued to increase, including 42,000 opioid-related overdose deaths in 2016. There is an urgent need to identify effective treatments and be aware of any collateral effects on the medical healthcare system. If a treatment for opioid use disorder has the unintended consequence of increasing healthcare utilization due to its antagonist effects on the brain (i.e., blocking a receptor rather than activating it like an agonist) or by lowering tolerance thereby increasing vulnerability to overdose during relapse, this critical information would need to be considered in policy and treatment planning. Extended release Vivitrol is an intramuscular injection that has emerged as a treatment alternative to traditional agonist therapies (e.g., Suboxone) for opioid use disorder, but not much is understood in the way it impacts hospital-based healthcare utilization. This study sought to fill that gap.
HOW WAS THIS STUDY CONDUCTED?
This was a secondary data analysis of a randomized clinical trial evaluating an injectable form of Vivitrol versus counseling and referral to community resources, including methadone, buprenorphine, and treatment programs (i.e., treatment as usual) in a criminal justice population of 308 participants. Participants received an initial injection of 380mg depot Vivitrol followed by subsequent injections every four weeks during the 6 month treatment phase. Follow-up visits occurred over the course of 12 months. The original study was conducted at five sites.
WHAT DID THIS STUDY FIND?
The authors found no difference on hospital-based healthcare utilizations including overall healthcare services (defined as number of emergency department visits and hospital admissions), drug detoxification, and psychiatric hospitalizations between the Vivitrol and treatment as usual groups with the exception of medical/surgical hospitalizations.
The overall effect of Vivitrol compared to treatment as usual is superior in terms of reducing the likelihood of medical/surgical hospitalizations during the first year and a half. In fact, patients diagnosed with opioid dependence were 45% less likely to have a medical/surgical hospitalization during the first year and a half when treated with extended release Vivitrol compared to treatment as usual. This 45% reduction is particularly notable given the Vivitrol group reported more chronic medical problems at baseline yet fewer medical/surgical hospitalizations during the study period. The benefit of Vivitrol did not extend to reduced utilization in terms of overall healthcare services, drug detoxification, or psychiatric hospitalizations (see Table 1). Although the authors did not speculate on why certain healthcare utilizations were reduced but not others, the reduction in medical/surgical hospitalizations may reflect that physicians were less likely to refer patients using Vivitrol with procedures that require medical or surgical admissions, since they may require opioid medications for acute pain management. Participants utilized a similar number of healthcare resources during that time with most patients reporting use of the emergency department or admission to the hospital less than 2 times during the entire 1.5 year study period.
Table 1: Effect of Injectable Vivitrol on Hospitable-Based Healthcare Utilization Among Patients with Opioid Dependence
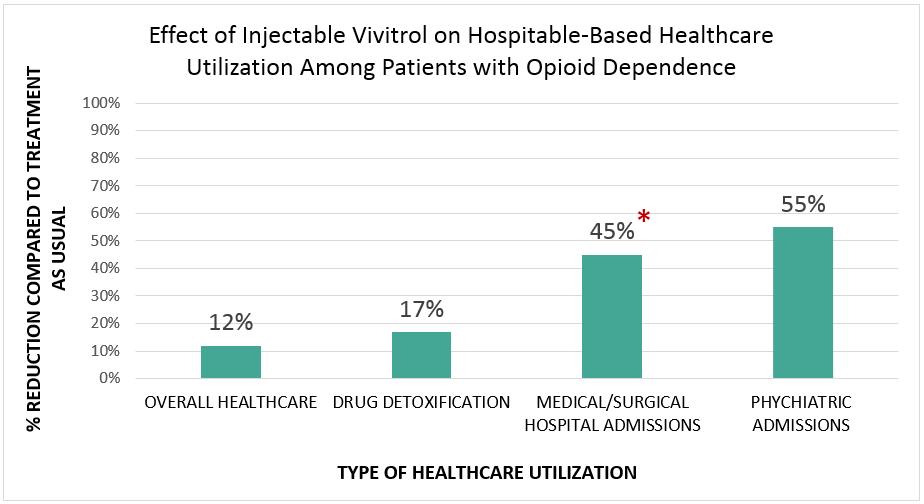
*Statistically significant difference.
Note. Although psychiatric admissions appear to be reduced by more than the
Medical/Surgical admissions the finding was none significant. Significance is
determined by a number of factors (standard deviation, overall sample size,
group sample size, mean, etc.) which made psychiatric admissions a
nonsignificant reduction.
WHY IS THIS STUDY IMPORTANT?
This study is important because it does not support the theory that healthcare utilization in the emergency department or inpatient hospitalization actually increase during treatment with extended release Vivitrol. Of the four trials that have evaluated extended release Vivitrol for the treatment of opioid use disorder, none reported on healthcare utilization as an outcome so this study provides new information regarding healthcare associated with extended released Vivitrol.
- LIMITATIONS
-
- The demographic of these participants was a criminal justice population who were either released from jail, plea bargained, or paroled. The degree to which these results are externally valid to non-criminal justice populations is unknown.
- The outcome variables were derived from self-report which subjects them to certain biases compared to data that is objectively derived from hospital and insurance records.
NEXT STEPS
Future research will need to determine the extent to which these healthcare utilization findings generalize to other populations including non-criminal justice populations and women with opioid use disorder. More research is needed to know if healthcare utilizations are the same among patients randomly assigned to receive Suboxone versus Vivitrol.
BOTTOM LINE
- For individuals & families seeking recovery:For the treatment of opioid use disorder, this study found that injectable Vivitrol did not cause increased healthcare utilization overall and was associated with a reduction in medical/surgical hospital admissions compared to counseling and referral to community resources including methadone, buprenorphine, and treatment programs. Other studies have found that injectable Vivitrol is similar to suboxone at helping reduce the risk of four or more consecutive weeks of non-medical opioid use, but only for patients who make it through the initial induction period, which can be difficult as this can be extremely uncomfortable for patients, and yet Vivitrol patients still have fewer days abstinent and fewer number of weeks opioid-free.
- For scientists:This study found no significant differences between a group of patients randomized to receive injectable Vivitrol versus treatment as usual in hospital-based healthcare resources including overall healthcare utilization, drug detoxification, or psychiatric hospitalizations. The Vivitrol group reported lower medical/surgical related hospital admissions during the course of the entire study despite having more chronic medical conditions at baseline.
- For policy makers:Extended release Vivitrol for opioid use disorder is associated with reduced medical/surgical hospital admissions compared to counseling and referral to community resources such as methadone, buprenorphine, and treatment programs (also known as treatment as usual). Furthermore, this study rebutted the hypothesis that extended release Vivitrol increases healthcare utilization. Given this medicine has been shown to be effective at reducing relapse when compared to placebo and to a lesser degree Suboxone (only for patients who make it through a potentially uncomfortable induction period) it is important to know more about its effect on medical healthcare utilization.
- For treatment professionals and treatment systems:This study found that extended release Vivitrol did not significantly increase rates of healthcare utilization compared to counseling and referral to community resources such as methadone, buprenorphine, and treatment programs. Clinician concerns regarding increasing healthcare utilization should not preclude the consideration of extended release Vivitrol as treatment for opioid use disorders.
CITATIONS
Soares, E. W., Wilson, D., Rathlev, N., Lee, J.D., Gordon, M., Nunes, E. V., O’Brien, C. P., & Friedmann, P. D. (2018). Healthcare utilization in adults with opioid dependence receiving extended release naltrexone compared to treatment as usual. Journal of Substance Abuse Treatment, 85, 66-69.

