A Real-world Comparison of Methadone & Buprenorphine for Opioid-dependent Pregnant Women
As the standard of care for treating opioid-dependent pregnant women for the past four decades, methadone maintenance has been proven to result in better health outcomes for both the mother and the newborn.
Buprenorphine (i.e., Subutex), a partial opioid agonist with less potential for misuse that is more easily accessible and requires less frequent medical follow-up than methadone, became available for use by pregnant women in 2004.
A randomized controlled trial (MOTHER) comparing the efficacy of the two medications in pregnant women found that buprenorphine was a viable alternative with a reduction in the severity of neonatal abstinence syndrome (NAS) among newborns.
Meyer and colleagues used data collected from 609 mother-newborn pairs treated for opioid dependence during pregnancy with methadone or buprenorphine between August 2000 and June 2012 to compare the effectiveness of the two medications in a real-world, clinical setting. Opioid agonist therapy (OAT) (i.e., methadone or buprenorphine) was determined by individual assessment and considered factors such as success with prior treatment, treatment availability, and patient choice.
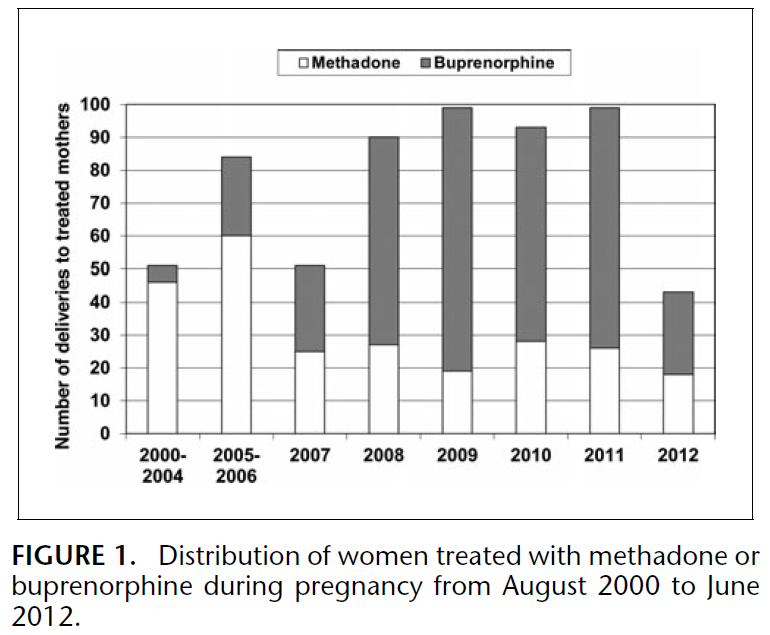
All patients were informed that methadone was the standard of care and were given the option to change medications if necessary. Since 19 women changed from buprenorphine to methadone during pregnancy, mothers were classified by opioid agonist therapy (OAT) type at time of conception. No women switched from methadone to buprenorphine. In this sample, 361 mothers were treated with buprenorphine and 248 mothers were treated with methadone at the time of conception. The figure below describes the distribution of pregnant women on OAT from 2000 to 2012.
Comparing significant differences in type of medication as a function of maternal characteristics, more first time mothers were treated with buprenorphine (36%) than methadone (23%) while a greater proportion of mothers treated with methadone were hepatitis C positive as compared to those treated with buprenorphine (41% vs. 26%). Both differences remained significant in the multivariate analysis adjusted for year of birth, maternal age, and smoking during pregnancy.
Regarding prenatal characteristics, a significantly larger proportion of mothers treated with buprenorphine reported adequate prenatal care (94% vs. 90%), a result that remained significant when controlling for the following: estimated gestational age at initial visit, BMI, pregnancy weight change, gestational age when OAT was initiated, year of birth, maternal age, and smoking during pregnancy. A significantly higher proportion of women on opioid agonist therapy (OAT) prior to conception were treated with buprenorphine (60%) as compared to methadone (40%). For women who began opioid agonist therapy (OAT) during pregnancy, on average, those using buprenorphine initiated treatment at an earlier gestational age than those using methadone (16 weeks vs. 19 weeks).
For newborn outcomes, treatment with buprenorphine as compared to methadone resulted in significantly longer gestational age, reduced incidence of preterm birth (defined as gestational age less than 37 weeks), larger birth weight, and larger head circumference. Newborns exposed to buprenorphine in utero needed medication for neonatal abstinence syndrome (NAS) significantly less often than those exposed to methadone (23% vs. 42%). The differences in gestational age, preterm birth, and neonatal abstinence syndrome (NAS) treatment remained significant in the multivariate analysis. Buprenorphine-exposed newborns had higher rates of breastfeeding than their methadone-exposed counterparts (75% vs. 63%).
IN CONTEXT
Consistent with an earlier randomized trial (i.e., MOTHER), this real-world study found important differences between treatment with buprenorphine and methadone in terms of prenatal characteristics and newborn outcomes.
Women treated with buprenorphine were more likely to initiate opioid agonist therapy (OAT) before or earlier in pregnancy and give birth at term to newborns that did not require treatment for neonatal abstinence syndrome (NAS).
An important distinction between this study and the MOTHER trial is that as an observational study, the authors were able to examine real world effectiveness of the medications rather than efficacy in a highly controlled setting where treatment was randomly allocated to participants. One difference between the two studies that favors clinical effectiveness of buprenorphine is that more women in the MOTHER study that were randomized to buprenorphine dropped out due to treatment dissatisfaction while only 3 women in this study changed to methadone for this reason. This finding shows that women in the clinical setting who are candidates for OAT are likely to achieve successful treatment with buprenorphine allowing the authors to conclude that buprenorphine is at least as favorable as methadone for the treatment of opioid-dependent pregnant women.
While conclusions regarding causality can be better drawn from the MOTHER study, the findings in this study help address the limitations of external and ecological validity in randomized controlled trials. Building upon the results found in the MOTHER study, this study supports the idea that buprenorphine may be a good choice for expectant mothers with opioid use disorders as the consequences for the newborn may be less severe than with methadone.
BOTTOM LINE
- For individuals & families seeking recovery: This study suggests if pregnant and using opioids, treatment with buprenorphine or methadone will be highly beneficial to the health of the mother and child. While buprenorphine is not the best option for every patient, it is worth considering as it is a safer, more convenient option.
- For scientists: An observational study investigating the effectiveness of buprenorphine for women who initiate OAT before conceiving (vs. those who start medication while already pregnant) could provide valuable information regarding optimal benefit of this medication for pregnant women and newborns.
- For policy makers: Since the benefits of OAT for pregnant women are consistently shown, it is crucial that maintenance therapy is available for pregnant women with opioid use disorders.
- For treatment professionals and treatment systems: Speak with your female patients who are pregnant or may become pregnant about initiating OAT since maintenance therapy has been proven to result in better outcomes for both mother and child than no treatment or medication-assisted withdrawal.
CITATIONS
Meyer, M. C., Johnston, A. M., Crocker, A. M., & Heil, S. H. (2015). Methadone and Buprenorphine for Opioid Dependence During Pregnancy: A Retrospective Cohort Study. Journal of addiction medicine.

