How can reported patient safety incidents improve opioid use disorder treatment with medication?
The increasing prevalence of opioid use disorder (OUD) and corresponding use of medications for this condition highlight the need for an OUD treatment system that is both safe and effective. In this study, authors characterized patient safety incidents that occurred in community-based OUD treatment over a 10-year period in England and Wales. How can these findings better inform systems of care in the United Kingdom and internationally?
WHAT PROBLEM DOES THIS STUDY ADDRESS?
The number of individuals who have an opioid use disorder (OUD) has increased since the turn of the century, fueled by the opioid crisis. Medications for OUD have emerged as an empirically-supported treatment option that decrease mortality, reduce illicit opioid use, and increase retention in treatment. Although OUD medications are underutilized, there has been an increase in the number of people prescribed these medications and an increase in facilities that offer this treatment option over the last 12 years. Therefore, it is increasingly important to identify strategies that lead to a better understanding of the OUD treatment system and better-informed policies and procedures.
There is variation in how OUD medications are delivered in the treatment system internationally. In the United States, methadone is provided at regulated and licensed Opioid Treatment Providers (OTPs), buprenorphine (which is also known by the common brand names Subutex or Suboxone when combined with naloxone) can be prescribed by healthcare professionals (e.g. physicians, physician assistants, and nurse practitioners) who have taken a special training course (i.e., they are “waivered”), and extended-release naltrexone (which is also known by the brand name Vivitrol) can be prescribed by healthcare professionals without any special training. Patients taking methadone may be required to initially go to the OTP on a daily basis where administration of the medication is supervised, whereas patients prescribed buprenorphine may pick up their prescription from a pharmacy monthly and patients on extended-release naltrexone will likely receive a monthly injection by their healthcare provider.
In the United Kingdom, where the current study takes place, OUD medications are delivered in community-based settings (i.e. pharmacies), there is no special training required by prescribers, and the medications are initially administered under supervision at the pharmacies. In this study, authors characterized patient safety incidents that occurred in community-based OUD treatment systems in England and Wales over a ten-year period in order to highlight any risks from the delivery of OUD medications that could lead ultimately to better-informed policies and procedures for the OUD treatment system.
HOW WAS THIS STUDY CONDUCTED?
This was a mixed-methods study (involving both quantitative and qualitative elements) that analyzed 2284 patient safety incidents involving opioid use disorder (OUD) treatment with methadone or buprenorphine over a ten-year period in England and Wales. The researchers aimed to identify and classify patient safety incidents related to OUD treatment with methadone or buprenorphine, describe and interpret themes from these incidents, and use this analysis to inform quality improvement in these systems of care. The authors used the National Reporting and Learning System (NRLS), a national database of patient safety incident reports compiled by the National Health Service (NHS) in England and Wales. A total of 272,884 patient safety incidents were identified during the time period 2005-2015 that were reported in community-based care settings (e.g. primary care and community pharmacies). Next, these patient safety incidents were narrowed down to only those involving methadone and buprenorphine (n=3297), then authors manually reviewed the reports, excluding those that had insufficient information, involved a non-OUD medication or used these OUD medications for pain management reasons, and did not meet criteria for a patient safety incident. The final analysis included 2284 patient safety incident reports.
A patient safety incident is defined as “any unintended or unexpected incidents that could have, or did, lead to harm for one or more patients receiving health care.” Each incident report requires the reporter to complete a form with categories that include location, type of incident, medication involved, and reporter’s perception of severity of harm as well as free text to describe the incident. Reporters can voluntarily complete free text sections on contributing factors and how the incident could have been prevented.

Figure 1.
The researchers used a World Health Organization (WHO) classification system, which includes a coding framework, to capture different aspects of the patient safety incident.
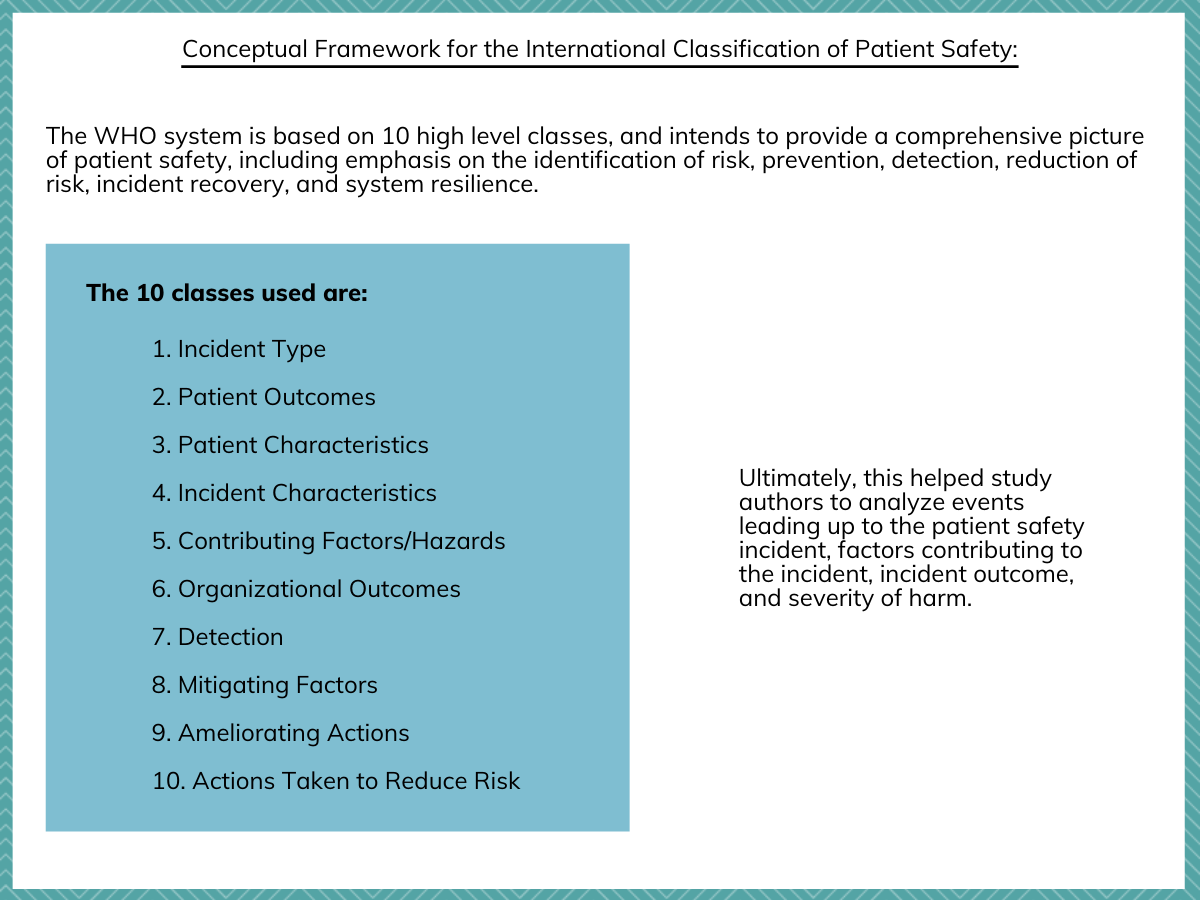
Figure 2. A description of the international patient safety incident classification that was used for the study.
Two researchers with clinical experience each were allocated half of the patient safety incident reports to read and code based on the WHO classification system. To minimize bias, each researcher also coded a random sample of 20% of the other researcher’s assigned reports, in which coding was deemed to be reliable. Discrepancies were discussed and resolved at regular meetings involving the research team. Exploratory data analysis was used to summarize the main characteristics of the patient safety incidents. Themes emerged and categories related to the process of care were identified.
WHAT DID THIS STUDY FIND?
Overall, reported harm from patient safety incidents was minimal and methadone was more commonly reported than buprenorphine.
Of the 2284 patient safety incidents that were analyzed, 82% involved methadone, 17% involved buprenorphine, and 1% involved buprenorphine with naloxone (also known as the brand name Suboxone). Ninety-four percent of patient safety incidents were perceived by reporters as causing no harm, 5% were perceived as causing low harm, and 1% were perceived as causing moderate harm, and this differed little across medication types. Notably, no patient safety incidents that were reported were perceived to result in severe harm or death.
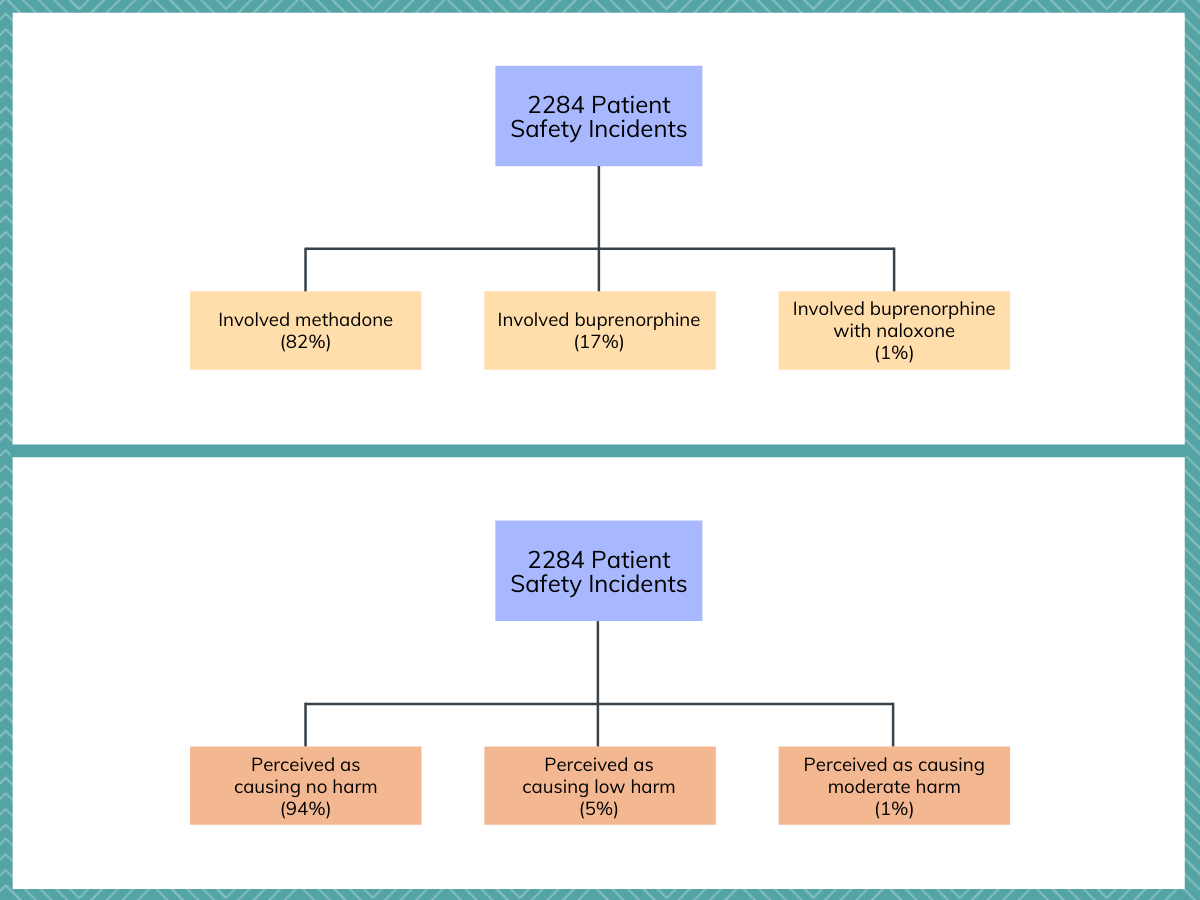
Figure 3.
Patient harm resulting from patient safety incidents differed among four processes of care.
Authors initially identified four processes of care responsible for most of the patient safety incidents. In decreasing frequency, these were non-supervised dispensing (68%), supervised dispensing (14%), monitoring and communication (11%), and prescribing opioid use disorder (OUD) medication (7%).
Incidents involving prescribing OUD medication were associated with the highest proportion of harm (9%), followed by monitoring and communication-related incidents (8%), failures in supervised dispensing (8%), and failures in non-supervised dispensing (4%).
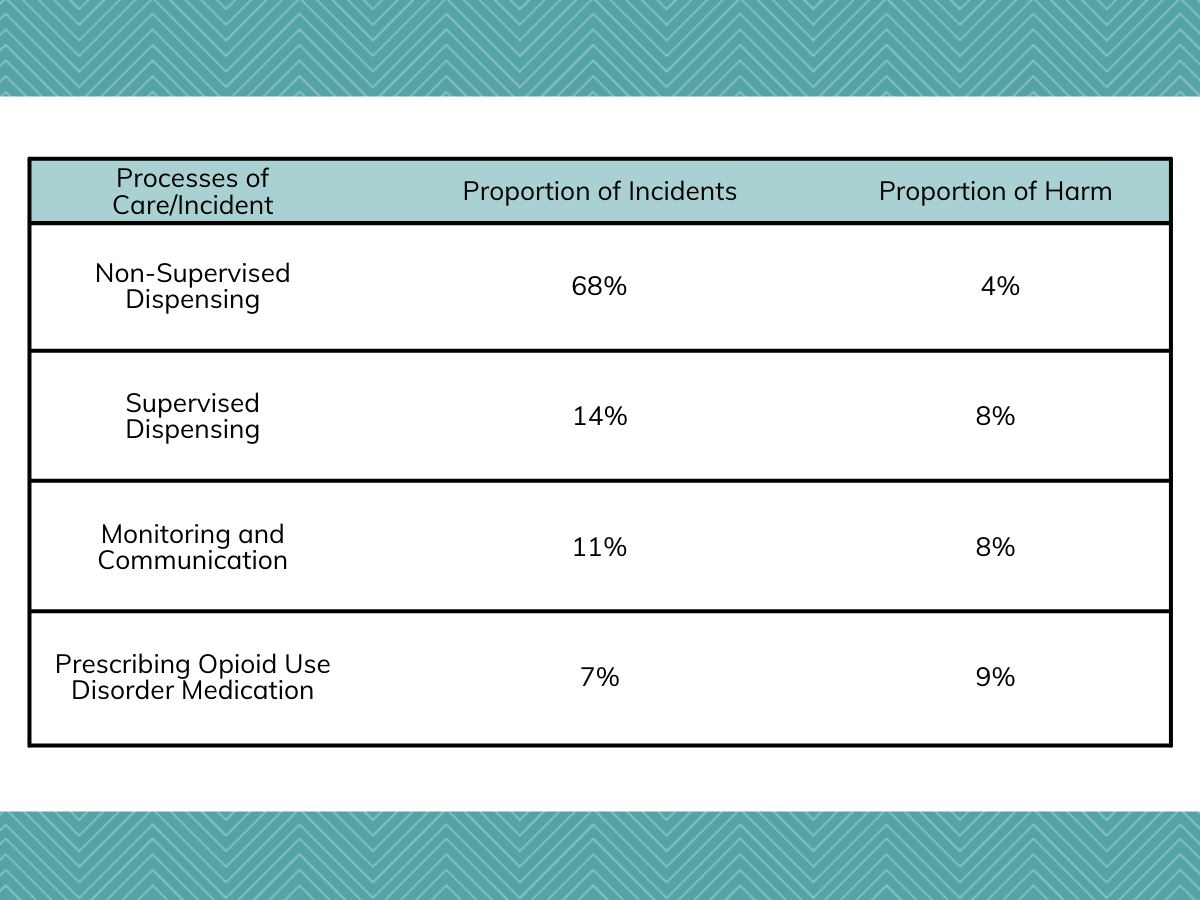
Figure 4.
Among each process of care, most incidents could be attributed to a few specific causes.
For the largest group of incidents that were related to non-supervised dispensing, most could be attributed to incorrect dose (23%), incorrect number of doses (20%), incorrect formulation (19%), dispensing medication to the wrong patient (14%), and dispensing medication against an invalid prescription (12%).
For supervised dispensing, most patient safety incidents could be attributed to dispensing medication to the wrong patient (31%), incorrect dose (23%), or failure to supervise consumption (19%).
For patient safety incidents that were related to monitoring and communication between the prescriber and the dispenser (e.g. pharmacist), most could be attributed to failure to stop dispensing the medication (30%), prescription handling incidents (23%), and duplicate prescribing (22%).
Lastly, for incidents involving prescribing OUD medication, most involved an incomplete/inaccurate prescription (39%) or a wrong dose written on the prescription (25%).
Causes that contributed to most of the patient safety incidents included both staff and organizational factors.
Staff factors were indicated in a large majority (85%) of patient safety incidents that included contributory factors followed by organizational factors (54%). Staff factors that were mentioned in the reports primarily included attentional slips by staff, such as being distracted or misreading the dosage of a medication. Organizational factors that were mentioned in the reports included poor working conditions and overwhelmed staff. Importantly, only 75% of the patient safety incidents had contributory factors listed by the reporter, and 40% of these reports listed more than one contributory factor.
WHAT ARE THE IMPLICATIONS OF THE STUDY FINDINGS?
This study showed that patient safety incidents involving the OUD medications methadone and buprenorphine were very low and occurred in four processes of care (non-supervised dispensing, supervised dispensing, monitoring and communication between prescriber and dispenser, and prescribing OUD medication), and were associated with staff and organizational factors, such as attentional slips and overwhelmed staff. As the prevalence of OUD has increased since the opioid crisis began at the turn of the century and more people are utilizing OUD medications, strengthening systems of care delivery that mitigate risks has become increasingly important.
Methadone was involved in more than four times as many patient safety incidents as buprenorphine. Even though it is likely that more patients are taking methadone, given that 41% of people receiving OUD medications in the United Kingdom were prescribed buprenorphine and 59% were prescribed methadone from 2010 to 2014, this fourfold difference is likely not entirely explained by more methadone patients. Previous studies have shown that, when comparing patients taking methadone and buprenorphine, they have similar retention in treatment (so long as the dose for buprenorphine is 16mg or greater) and similar mortality rates. Thus, given the likely higher rate of patient safety incidents attributed to methadone, buprenorphine may be a safer option that is just as effective as methadone for some individuals.
Many of the patient safety incidents were attributed to incorrect dosages or formulations of medications, the wrong patient receiving medication, or miscommunication between prescriber and dispenser. In addition, a large majority of the perceived contributing factors to the patient safety incidents were related to attentional slips and overwhelmed staff. A centralized system of electronic health records (EHR) could improve information sharing between the prescriber and the dispenser, decrease attentional slips and medication errors with the use of safety prompts, and increase staff efficiency and productivity, thus mitigating many of causes and contributing factors of patient safety incidents involving methadone or buprenorphine. An extra layer of security should be implemented to ensure the privacy and confidentiality of patients on OUD medications while preserving coordination of care.
Notably, several incidents that posed potential harm to the patient were prevented by the patients themselves, such as noticing that an incorrect dosage had been prescribed. This highlights the role of the patient in identifying and preventing safety incidents. Patient-centered care involves shared decision-making, where patients are actively involved in their treatment plans and are educated about risks of treatment, thus strengthening the patient-provider relationship and giving patients autonomy to counteract failures in the treatment system. Patient-centered care could also increase retention in treatment, which is critical in improving outcomes.
Generalizing the study findings to the U.S. delivery system for OUD medications should be done cautiously. There are notable differences between the U.S. and U.K. systems, as buprenorphine is delivered similarly to the system in England and Wales, though no supervision is required at a pharmacy, whereas methadone is delivered through licensed and regulated Opioid Treatment Providers (OTPs). However, regulatory changes have been advocated for in the U.S. that align with the OUD treatment system in the United Kingdom.
As such, given that non-traditional routes to initiate and engage people on OUD medications have emerged in the U.S., including the criminal justice system, community health centers, as a post-overdose response in emergency departments and through outreach programs, findings here might help inform these new points of access. Specifically, it is possible that as more staff are involved in an individual’s care, there might be increased risk for human error, resulting in patient safety incidents. Research like this can inform policies and procedures to prevent human error, thereby ensuring a safer OUD medication treatment system.
It is notable that only 6% of patient safety incidents were reported as low or moderately harmful, and no incidents were classified as causing severe harm or leading to death. This data, taken over a ten-year period in two countries (England and Wales), may lead one to believe that methadone and buprenorphine are generally safe. However, this type of data should not be used to make estimates of the overall prevalence of incidents in a system due to under-reporting and reporter bias. Patient safety incidents involving no harm or minor harm may be underreported due to the time-intensive process of completing the form whereas patient safety incidents involving moderate or severe harm may be underreported due to staff fearing personal blame and its associated consequences. In addition, patients who experience incidents that result in severe harm are likely to present to an emergency department, and thus would likely not be captured by the database used in this study.
On the contrary, this type of aggregated data at the national level is most useful in offering important insights into systemic factors that underly patient safety incidents and associated harms. Future studies with more intensive follow up of patient safety incidents, such as retrospectively viewing patient notes and linking to multiple databases (such as claims, hospital records, or death data), may give a better overall estimate of these patient safety incidents in the OUD treatment system.
- LIMITATIONS
-
- There may be under-reporting of patient safety incidents and reporter bias present. Thus, estimation of the overall prevalence of these incidents over the time period and interpretations from this estimation can be misleading.
- Even though there was no severe harm caused by these reported incidents over the time period, this could be because of reporter bias (i.e. the person reporting the incident underestimated the harm involved) or unreported patient safety incidents associated with severe harm or death did not show up in the database as these patients would be more likely to show up in the emergency department, not community-based clinics.
- The database used did not include the variables used in this study. Instead, authors derived these variables by making decisions on how to code quantitative and qualitative data, which can lead to observer bias. Measures were taken to minimize this bias, such as regular coding meetings, double-coding, and coding by researchers with clinical experience.
- The setting of this study was England and Wales. Implications of the study findings may not be generalizable to other countries with different opioid use disorder treatment systems, such as the United States.
- The voluntary, open-ended questions, such as contributing factors, were only filled out in some of the patient safety incident reports, potentially limiting findings.
BOTTOM LINE
- For individuals and families seeking recovery: This study showed that patient safety incidents involving the opioid use disorder (OUD) medications methadone and buprenorphine occurred in four processes of care (non-supervised dispensing, supervised dispensing, monitoring and communication between prescriber and dispenser, and prescribing OUD medication), and were associated with staff and organizational factors, such as attentional slips and overwhelmed staff. Notably, this study found that several patient safety incidents that posed potential harm were prevented by the patients themselves. Patients and families can benefit from being educated on and involved in the treatment process. This increased patient autonomy can prevent medication errors and help cultivate the patient-provider relationship.
- For treatment professionals and treatment systems: This study showed that patient safety incidents involving the opioid use disorder (OUD) medications methadone and buprenorphine occurred in four processes of care (non-supervised dispensing, supervised dispensing, monitoring and communication between prescriber and dispenser, and prescribing OUD medication), and were associated with staff and organizational factors, such as attentional slips and overwhelmed staff. Many of the safety incidents were related to medication errors or miscommunication between the prescriber and the dispenser. A centralized system of electronic health records (EHR) with safety prompts could mitigate many of these risks to the patient. In addition, patients themselves prevented harm from some of the safety incidents, highlighting the importance of shared decision-making and cultivating the patient-provider relationship.
- For scientists: This study used a national database of patient safety incidents in England and Wales over a ten-year period to identify incidents involving the opioid use disorder (OUD) medications methadone and buprenorphine, and classified them into four processes of care (non-supervised dispensing, supervised dispensing, monitoring and communication between prescriber and dispenser, and prescribing OUD medication). Contributing factors of these incidents were related to staff and organizational factors, such as attentional slips and overwhelmed staff. Notably, patient safety incidents involving methadone were more than fourfold higher than those involving buprenorphine, and only 6% of reported incidents were perceived as low or moderately harmful, with none associated with severe harm or death, over a ten-year period. Due to the limitations of this study, more research is needed using different research designs to confirm conclusions from these findings. Also, the type of incidents reported highlight the need for a centralized system for electronic health records (EHR), including strategically placed safety prompts, to mitigate risks to patients.
- For policy makers: This study showed that patient safety incidents involving the opioid use disorder (OUD) medications methadone and buprenorphine occurred in four processes of care (non-supervised dispensing, supervised dispensing, monitoring and communication between prescriber and dispenser, and prescribing OUD medication), and were associated with staff and organizational factors, such as attentional slips and overwhelmed staff. Study findings highlight that one potential way to mitigate these incidents across the processes of care is to implement a centralized system of electronic health records (EHR) with safety prompts. This could be done through legislative mandates, incentivizing implementation through alternative payment models, or funding EHR infrastructure in these treatment systems. The types of incidents and contributing factors identified by this study could inform policies and procedures in the OUD treatment system, especially in a system of care delivery that involves a prescription from a provider and dispensing of the medication from a community-based pharmacy.
CITATIONS
Gibson, R., MacLeod, N., Donaldson, L.J., et al. (2020). A mixed-methods analysis of patient safety incidents involving opioid substitution treatment with methadone or buprenorphine in community-based care in England and Wales. Addiction, [Epub ahead of print]. doi: 10.1111/add.15039

