Can neuroimaging be used to help us understand the brain during opioid use disorder recovery?
The widespread use of prescription opioids and heroin in the United States during the past decade, along with the emergence of illicit fentanyl, has resulted in an unprecedented epidemic of opioid overdose deaths and an urgent need to develop, test, and disseminate opioid use disorder treatments. Modern neuroimaging technology is advancing our ability to measure and quantify how the brain is changed during substance use disorder and recovery from substance use problems. In this study, authors review neuroimaging research that could identify potential new targets for the treatment of opioid use disorder.
WHAT PROBLEM DOES THIS STUDY ADDRESS?
In the past decade, there has been an unprecedented spike in opioid use disorder, which has led to more than 300,000 opioid-related deaths in the United States. The neuroscience underlying opiate addiction is not well-understood. The authors of this study review brain circuitry and processes associated with opiate addiction within the context of a Three-Model Theory of Addiction, characterizing processes of 1) binge/intoxication; 2) negative reinforcement (i.e., drug-taking to reduce unpleasant physical and emotional states); and 3) anticipation and preoccupation. Authors then discuss strengths and limitations of this imaging work and suggest future research that could help researchers and treatment professionals use neuroscience to inform potential points for interventions.
HOW WAS THIS STUDY CONDUCTED?
The authors conducted a narrative review of the neuroimaging studies that have been conducted in those with opioid addiction. The authors divide this research according to three stages of addiction: “Binge/Intoxication,” the stage at which a person uses a drug for its rewarding, feel-good properties, “Negative Reinforcement,” the stage at which a person uses a drug to stave off withdrawal, stress, and negative emotions produced by taking the drug over a long period, and “Preoccupation/ Anticipation,” the stage at which a person looks forward to using the drug again and uses the drug compulsively, as drug use becomes more and more difficult to control. Within this model, these stages are linked to specific patterns of activity within/between brain regions involved in reward processing (ventral striatum), cognitive control and decision-making (prefrontal cortex) negative emotional states (amygdala), among other brain regions important in addiction.
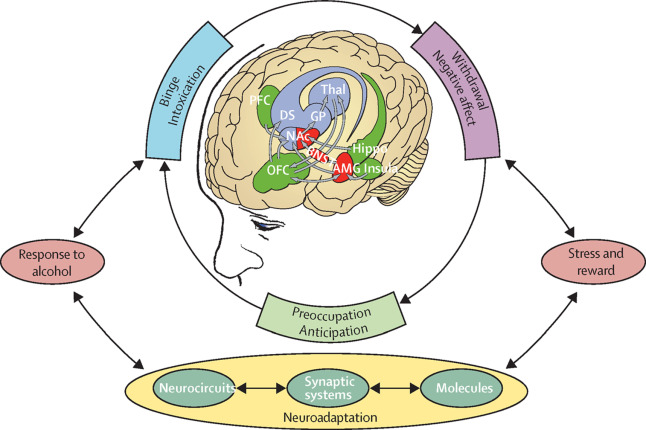
Figure 1. Three-Model Theory of Addiction Neurobiology as contextualized by the study authors for the purpose of this review. Image taken from Volkow and Koob, 2015.
WHAT DID THIS STUDY FIND?
This narrative review explains how brain differences have important behavioral consequences. In the Binge/Intoxication stage, neuroimaging studies confirm that the key brain region in reward processing, the ventral striatum, shows differences in its “activation” (e.g., how much oxygen is being used in the brain) when individuals with opioid addiction (vs. those without addiction) are looking at drug cues of opioid-related images. During the Negative Reinforcement stage, studies show that the connection between the amygdala (involved in the processing of negative emotional states) and the ventral striatum (involved in the processing of reward) is stronger in those with opioid addiction compared to those without opioid addiction, indicating an unhealthy relationship between reward and negative affect in those with addiction.
During the Preoccupation/Anticipation stage, the prefrontal cortex – the brain region that is most important in exerting control over one’s behavior – shows increased activation to drug cues and decreased activation to other rewarding cues (food, sex cues), perhaps explaining why it is so difficult for those with opioid addiction to stop using and drug use taking on such an abnormally high priority in the person’s life to the exclusion of other natural rewards. Increased reward activation in the ventral striatum predicts drug relapse, and increased activation of the prefrontal cortex predicts treatment adherence and effects of medications, such as naltrexone, which are used to treat opioid addiction.
The authors discuss longitudinal neuroimaging studies of opioid addiction, which combine neuroimaging data with treatment outcomes, to examine changes in the brain with abstinence. This research primarily focuses on brain responses to drug cues.
Neuroimaging studies indicate that greater brain activity in the prefrontal cortex to opioid images predicts greater opioid use frequency 6 months later, whereas lower brain activity during cognitive control tasks predicts future treatment discontinuation. These findings point to executive function/decision-making deficits within the Preoccupation/Anticipation stage, as individuals who relapse discount natural rewards (e.g., food, sex, enjoyable social activities) and prioritize drug-related reward. Also, greater ventral striatum response (paired with higher self-reported “craving” for opiates) to opioid cues predicts relapse within 3 months, whereas greater prefrontal cortex activation to opioid cues predicts more successful treatment outcomes. Additionally, studies examining how brain regions are “connected” to one another (e.g., functional connectivity) demonstrate that greater connectivity between the ventral striatum and prefrontal cortex predicts successful substance use treatment. In summary, these findings indicate that heightened brain activity to drug cues (particularly in striatal “reward” and frontal “control” regions) forecasts difficulty maintaining sobriety. Together, these studies underlie the reality that addiction changes the brain in fundamental ways, which makes it difficult, though certainly not impossible, to achieve long-term sobriety.
WHAT ARE THE IMPLICATIONS OF THE STUDY FINDINGS?
These important brain changes throughout the course of opioid addiction can have implications for treatment. For example, naltrexone shows promise in reducing brain activity to drug-related cues, which suggests that naltrexone, and potentially other treatments, can change the way the brain functions. Extended release, injection naltrexone (e.g., vivitrol) can be life-saving for some patients, and perhaps this brain data showing that it can reduce activity to drug cues offer a neurobiological explanation for why vivitrol is helpful. Other studies show that individuals with opioid addiction completing 4 weeks of mindfulness-based treatment show improved functional connectivity between the striatum and frontal regions, which point to an improvement in how the brain’s reward areas are connected to its high-order decision-making regions. The types of patterns in the brains of those with opioid use disorder highlighted in this paper are also present across a range of substance use disorders (SUD). For example, in a study summarized in a prior issue of the RRI Recovery Bulletin, authors looked at the brains of individuals with chronic cocaine use disorder when they chose long-term rather than short-term rewards, and found that those with cocaine use exhibited greater activity in a region called the dorsal striatum. Though neuroscience studies have not yet been directly translated into treatment, this study shows that there is imaging technology available to look into the brain, and that such technology may one day inform treatment matching to determine the likelihood of an individual responding well to specific types of interventions.
- LIMITATIONS
-
- Researchers still have very limited knowledge about the complexities of the brain, and this makes it hard to take general findings (which are often presented at a group level – e.g., those with opioid addiction vs. those without) and apply them to individual patients.
- Many of the studies referenced in this review were small and cross-sectional, meaning that they didn’t assess individuals before they began using opioids and again after opioid use. Therefore, many of these studies do not demonstrate that opioid use disorder caused the observed brain changes, or, in parallel, that the treatments highlighted caused any differences between treated and untreated individuals.
- Neuroimaging studies often are too small to look at important subgroups, such as women, people with specific psychiatric comorbidities (e.g., depression), or trauma-exposed individuals. Thus, it is uncertain how these results may apply to each of these important subgroups.
- Currently, we do not know whether information collected in brain scans can predict, at an individual patient level, with sufficient specificity to guide individual treatment recommendations. More research needs to be done in order to better understand whether there is clinical utility in brain scans.
BOTTOM LINE
- For individuals and families seeking recovery: This narrative review shows that neuroimaging may help explain brain changes with opioid addiction. It may be comforting for patients and their families to understand and be able to visualize, by examining images of the brain, that addiction is a brain disease, and like many other diseases, can recover with treatment. Neuroimaging studies that generate multiple images of the brain over time can help shed light on how the brain changes in the context of substance use disorder treatment and recovery. Brain images also provide opportunities to identify brain differences that are “biomarkers” (i.e., observable signs based on a test of physical or other medical functioning) for worse treatment outcomes. Identifying these at-risk individuals can provide opportunities to modify their treatment based on their unique needs, often called “personalized medicine.” It is important to note, however, that we do not know whether information collected in brain scans can predict, at an individual patient level, with sufficient specificity to guide individual treatment recommendations. More research needs to be done in order to better understand whether there is clinical utility in brain scans.
- For treatment professionals and treatment systems: This narrative review demonstrates that neuroimaging is a promising tool to explore brain mechanisms that are affected by addiction and can potentially be used to understand brain responses to treatment. Although translating individual patients’ brain scans into predictors of treatment is not yet feasible, as our understanding of the brain increases and as technology becomes more advanced, it is certainly possible that brain scans will someday be used in the clinic. Identifying at-risk individuals can provide opportunities to modify their treatment based on their unique needs, often called “personalized medicine.”
- For scientists: This narrative review shows that neuroimaging may help uncover and explain the brain changes associated with opioid addiction. Neuroimaging studies provide another dimension to recovery research. Identifying biomarkers and predictors of substance use outcomes may eventually help inform clinical practice. It would be helpful for neuroimaging scientists to continue to examine whether brain scans are clinically useful, as well as to study different subgroups such as women, people with specific psychiatric comorbidities (e.g., depression), or trauma-exposed individuals, in order to better understand how differences in the brain may translate to substance use outcomes.
- For policy makers: This narrative review shows that neuroimaging may help uncover and explain the brain changes associated with opioid addiction. There is inherent value for policymakers to be able to see brain changes with addiction, similar to how one would visualize an x-ray of a broken bone, in order to further destigmatize addiction, with the goal of enhancing the effectiveness of treatment. Neuroimaging can be used to make the case that treatment changes the brain and can help make the case that treatment is an effective use of fiscal resources. Allocating funding to examine this possibility – that brain scans may help to identify at-risk individuals – might help provide answers on the utility of brain image assessments, and, subsequently, what should be done to help intervene if and when biomarkers of relapse risk are discovered.
CITATIONS
Stewart, J. L., May, A. C., Aupperle, R. L., & Bodurka, J. (2019). Forging neuroimaging targets for recovery in opioid use disorder. Frontiers in Psychiatry, 10(117), (Epub). doi: 10.3389/fpsyt.2019.00117

