Which Medications are Best for Individuals That Want to Moderate Their Drinking?
The idea of giving people with alcohol use disorder medication to help them reduce or moderate their drinking is a relatively new concept. This paper reviewed the research to date to determine which medications, if any, are effective for helping individuals with alcohol use disorder moderate their drinking.
WHAT PROBLEM DOES THIS STUDY ADDRESS?
Alcohol use disorder kills approximately 88,000 Americans annually, and exacts a prodigious economic toll of around $249 billion each year in medical expenses, loss of productivity, accidents, and crime. Helping individuals with alcohol use disorder to achieve abstinence-based recovery can be a long and challenging process. Recognizing this, many clinical scientists and clinicians support the use of harm-reduction strategies. For individuals with alcohol use disorder a commonly employed harm reduction strategy is to help patients reduce their drinking, even though abstinence might be the patient and/or treatment provider’s ultimate aim. Though perhaps counter-intuitive, harm reduction strategies such as this can be very effective at reducing health burden, and can be an important step on the path to abstinence-based recovery for many people with alcohol use disorder.
Typically, harm reduction strategies are taught to patients by clinicians and counselors in the context of psychotherapy. There is a growing trend, however, to prescribe individuals with alcohol use disorder medications that will diminish their desire to drink, thus reducing their alcohol intake. The medications employed to do this are usually those already approved for, and/or used to treat addiction. Palpacuer and colleagues conducted a systemic review of published and unpublished papers testing these various medications to gauge whether they have the capacity to reduce alcohol consumption in individuals with alcohol use disorder.
HOW WAS THIS STUDY CONDUCTED?
The authors conducted a systematic review of double-blind randomized controlled trials (the gold-standard research design) of medications commonly used to help people with addiction achieve alcohol and/or other drug abstinence including, nalmefene, naltrexone, acamprosate, baclofen, and topiramate, to assess the capacity of these medications to reduce alcohol use among individuals with alcohol use disorder.
- MORE ON STUDY METHODS
-
To be eligible for inclusion in the review, studies needed to involve adults (aged 18 years and over) with a diagnosis of alcohol use disorder (as stipulated in the Diagnostic and Statistical Manual of Mental Disorders 5; DSM-5), or alcohol dependence (as defined by the Diagnostic and Statistical Manual of Mental Disorders IV; DSM-IV). Since the authors focused their review on non-abstinent patients, and detoxification is generally obtained after 5–7 days, they only included patients with fewer than 5 days’ abstinence before the beginning of the study. Therefore, studies were not included when longer abstinence (or detoxification) was an explicit inclusion criterion. Studies with patients presenting with co-occurring systematic physical or psychological conditions (e.g., head trauma, psychotic disorder) were also not included. Studies were eligible if they focused on the comparison of oral formulations (i.e., pills taken by mouth) of nalmefene, naltrexone, acamprosate, baclofen, or topiramate with each other or with a placebo. Only studies using a single medication (i.e., monotherapies) were considered. In case of studies which utilized multiple medication doses, only the dose closest to the recommended dose was taken into account. Only studies reported in English, French, German and Spanish were considered.
Papers were found by searching online scientific article databases including PubMed/Medline, the Cochrane Library, Embase, ClinicalTrials.gov, as well as websites for organizations such as the Food and Drug Administration and the European Medicines Agency. Pharmaceutical companies were also contacted and asked to provide unpublished findings. After paper identification, screening, and eligibility determination, 32 articles remained and were utilized by the authors. All studies reviewed included a placebo condition, and were published between 1994 and 2015.
In addition to reviewing the written content of identified papers, the authors conducted a meta-analysis (one large analysis of results from individual studies) of reported results to determine if, taken together, findings statistically support the use of these medications to reduce alcohol consumption among individuals with alcohol use disorder. The authors also independently assessed the risk of bias (e.g., studies that failed to consider outcomes for individuals who dropped out of a study because of medication side-effects) in each study using the Cochrane Collaboration tool for assessing risk of bias. This means we can have greater confidence in the results from the studies included in the authors’ review.
The studies reviewed compared the effects of oral nalmefene (n = 9), naltrexone (n = 14), acamprosate (n = 1), baclofen (n = 4), or topiramate (n = 4) against placebo. No study provided direct comparisons between these medications. Nalmefene and naltrexone were the most widely studied drugs with, respectively, 1693 and 850 patients receiving one of the two medications in the trials. The other drugs were studied less frequently with, respectively, 258, 106 and 349 patients for acamprosate, baclofen and topiramate. Generally, study durations were longer for nalmefene studies (median: 24weeks) than for naltrexone (median: 12 weeks), baclofen (median: 12 weeks) and topiramate (median: 12weeks). Except for one nalmefene trial and one naltrexone trial, all patients received a psychological co-intervention (including medical management programs) during the study periods.
WHAT DID THIS STUDY FIND?
RISK OF BIAS WITHIN STUDIES
The risk of bias assessment is reported graphically below. Twenty-six studies (81%) were classified as having an unclear or a high risk of incomplete outcome data due to a large number of study participant withdrawals. Seventeen studies (53%) were considered to present an unclear or a high risk of selective outcome reporting, as they did not mention a protocol registration number that allowed the authors to check whether all outcomes were reported.
Interpreting the image: The more red in a quadrant, the more risk of bias. The more risk of bias, the less confidence we have in the validity of the results.
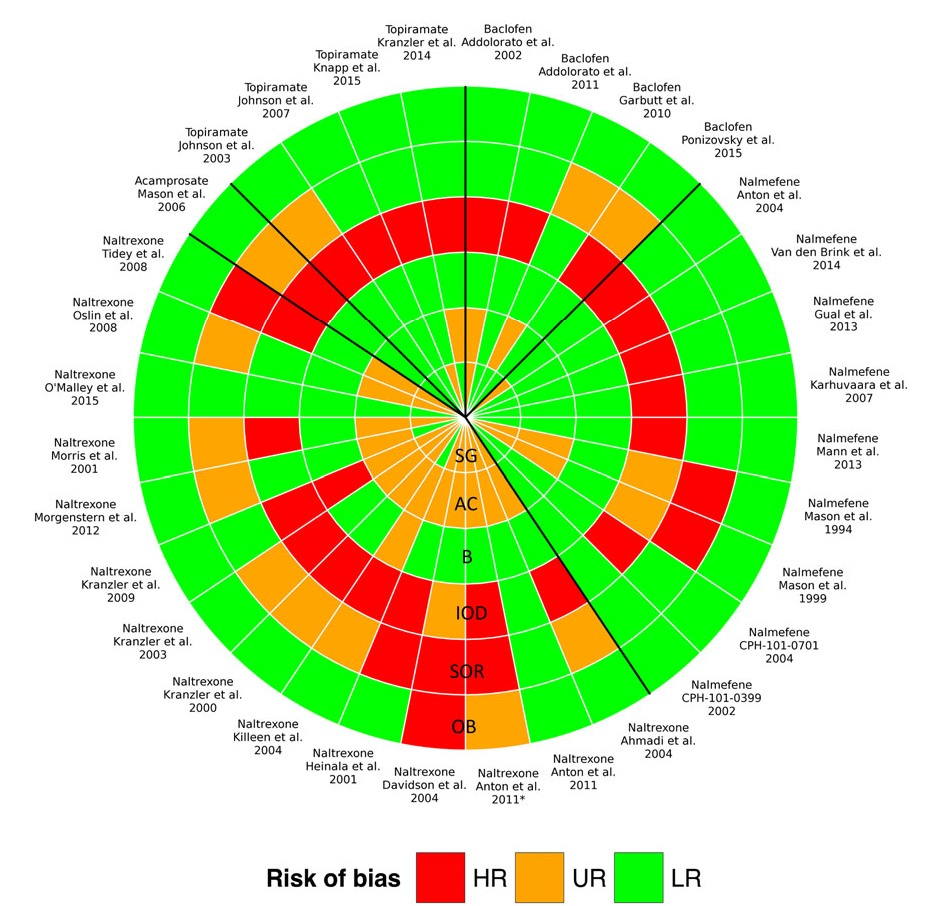
DIRECT COMPARISONS OF MEDICATIONS TO PLACEBO
The authors’ primary outcome, total alcohol consumption (TAC), was reported in only seven (78%) nalmefene studies, five (36%) naltrexone studies, one (100%) acamprosate study, one (25%) baclofen study and two (50%) topiramate studies. Nalmefene, topiramate and baclofen showed a greater decrease in TAC than placebo. Data on heavy drinking days (HDD) were more complete. The same results were observed, except for baclofen, which do not do any better than placebo. Compared to placebo, topiramate increased significantly (i.e., reliably, unlikely due to chance) the number of non-drinking days, and reduced the number of drinking days. Nalmefene reduced significantly the number of drinks per drinking days. For nalmefene, estimated effect sizes were always small, meaning someone getting this medication would most likely experience a relatively small benefit over placebo. Conversely, the effect size for baclofen was large on TAC but inconsistent on other criteria. Topiramate consistently showed medium to large effect sizes on all consumption outcomes. No difference was found for any drug on mortality and serious adverse events. More adverse events and withdrawals for safety reason were evidenced for naltrexone and nalmefene, and more withdrawals for nalmefene.
INDIRECT COMPARISONS OF MEDICATIONS TO ONE-ANOTHER
As shown in the figure below, for Total Alcohol Consumption (TAC), based on the evidence detailed for direct comparisons, topiramate was superior to nalmefene, naltrexone and acamprosate. Similarly, baclofen showed superiority over naltrexone and acamprosate on TAC. There was no evidence for a difference between nalmefene and naltrexone. The superiority of topiramate seemed to be consistent on all the other consumption outcomes while the superiority of baclofen was not. No significant difference was found across drugs on the safety outcomes, except for withdrawals for safety reasons. Overall, medications more likely to reduce TAC also had poorer safety profiles.
Interpreting the image: Green indicates that the treatment was most effective, while red indicates least effective.
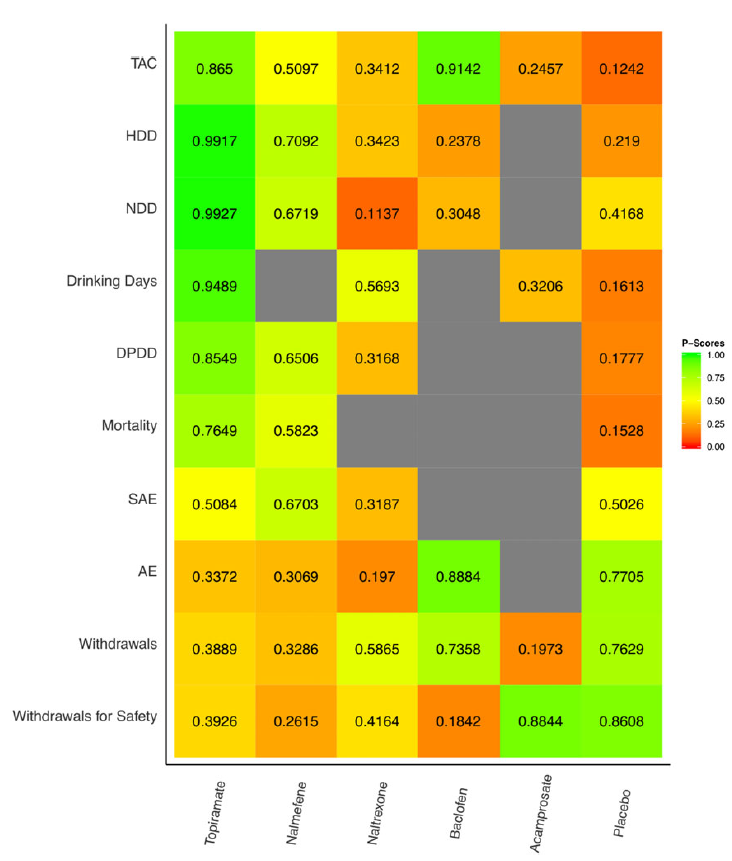
CONCLUSIONS
Regarding the primary outcome (total alcohol consumption; TAC), nalmefene, baclofen and topiramate showed superiority over placebo. Furthermore, indirect comparisons showed superiority of topiramate over nalmefene, naltrexone and acamprosate. It is noteworthy that the reporting of TAC was less consistent than the reporting of other consumption outcomes such as heavy drinking days (HDD). However, similar trends were observed for the other consumption outcomes, except in the case of baclofen (the positive results for baclofen on TAC were based on a single small study). For all treatments except topiramate, effect sizes were small or inconsistent. Furthermore, nalmefene and naltrexone were associated with a significant increase in withdrawals from the study and withdrawals for safety reasons, which raises concerns about a plausible attrition bias in these studies.
Though there were observed differences in effects between these medications, the authors conclude that there is currently no high-grade evidence for pharmacological treatment to control drinking using nalmefene, naltrexone, acamprosate, baclofen or topiramate in patients with alcohol dependence or alcohol use disorder. They qualify this position by noting that drinking reduction effect sizes were at best low to medium in size, and most of the studies reviewed had a high risk of bias. They also note that overall, there is no evidence of a significant association between these medications and reductions in serious alcohol-related adverse events or in mortality. This is largely because the reviewed studies were aimed at helping participants maintain alcohol abstinence, and no study was large or long enough to investigate these health outcomes.
WHY IS THIS STUDY IMPORTANT
Medications to help individuals with alcohol use disorder achieve abstinence have been around for some time. The idea, however, of utilizing these medications to reduce alcohol use among individuals with alcohol use disorder, who are perhaps not ready to attempt alcohol abstinence, is relatively novel. This study is important because it systemically and critically weighs for the first time research that speaks to the effects of nalmefene, naltrexone, acamprosate, baclofen, and topiramate on alcohol use reduction in individuals with alcohol use disorder. The authors’ findings suggest at least some of these medications have promise for this purpose, although as noted, more research is needed before any real conclusions can be drawn. The authors provide important suggestions about the future directions this research should take.
- LIMITATIONS
-
- Indirect comparisons between studies rely on the assumption that the studies being compared are similar in design/structure. Therefore, systematic differences such as sample composition, length of treatment etc. might bias meta-analysis results.
- Although the authors carefully selected studies including non-abstinent patients, different treatment goals across studies could have affected results. As the authors note, patients engaged in abstinence-orientated treatment may be likely to stop taking the drug when they begin drinking because they see this as treatment failure. The exact treatment goals in the reviewed studies were reported inconsistently, and it was therefore not possible for the authors to take this information into account.
- Because nalmefene is an approved medication in Europe, the European Medicines Agency granted the authors access to unpublished studies and study reports; therefore, to the authors’ knowledge, all available relevant data from completed studies on nalmefene that met their inclusion criteria were included in their review. This was not the case for the other medications. When looking at fewer studies, the medication effects may appear to be larger than they are in reality due to natural fluctuations in study findings depending on the study characteristics. As more studies are included, effects tend to decrease, but become more reliable approximations of the true effects of the medication. Therefore, obtaining all studies conducted on nalmefene but only some for the other medications could have biased results towards an underestimation of the comparative effectiveness of nalmefene.
NEXT STEPS
Researchers and stakeholders need to establish a coherent agenda to demonstrate that pharmacologically controlled drinking can be translated into genuine harm reduction for patients. This will include large-scale, longitudinal, randomized-controlled trials that minimize bias, and explore health outcomes (including large, randomized, controlled cluster trials) in targeted populations. In addition, research is needed to better understand for whom these medications are effective. It is known for instance that individuals carrying certain genes (e.g., OPRM1 Asp40) show a better response to Naltrexone in terms of alcohol use reduction compared to those not carrying these genes.
BOTTOM LINE
- For individuals & families seeking recovery: Currently, the evidence supporting the use of nalmefene, naltrexone, acamprosate, baclofen, and topiramate to help individuals with alcohol use disorder reduce their drinking is mixed. While these medications may be effective for some people, overall reductions in alcohol use were found to be fairly small, and adverse medication side effects are a concern. Although the evidence for these medications’ ability to reduce alcohol use is currently mixed, these medications should not be discounted by individuals seeking alcohol abstinence, as previous research has shown these medications to help individuals with alcohol use disorder achieve abstinence-based recovery.
- For scientists:There is currently no high-grade evidence for pharmacological treatment to control drinking using nalmefene, naltrexone, acamprosate, baclofen or topiramate in patients with alcohol dependence or alcohol use disorder. Some treatments show low to medium efficacy in reducing drinking across a range of studies with a high risk of bias. None demonstrates any benefit on health outcomes. More work is needed to determine for whom these medications are most effective (i.e., genotypes). Studies are also needed testing these medications with individuals who are specifically attempting to reduce their drinking, rather than achieve abstinence.
- For policy makers: Although the present findings suggest limited effectiveness of nalmefene, naltrexone, acamprosate, baclofen, and topiramate to help individuals with alcohol use disorder reduce their drinking, these medications have been shown to help individuals with alcohol use disorder achieve alcohol abstinence. Policies facilitating patient access to these medications are likely to have a significant public health benefit
- For treatment professionals and treatment systems: Currently, the evidence supporting the use of nalmefene, naltrexone, acamprosate, baclofen, and topiramate to help individuals with alcohol use disorder reduce their drinking is mixed. While these medications may be effective for some people, overall reductions in alcohol use were found to be fairly small, and adverse medication side effects are a concern. These findings do not preclude the fact these medications may be effective for helping some individuals reduce their drinking. However, given the generally small to medium effect sizes associated with these medications, they should not be considered stand-alone treatments for reducing alcohol use in individuals with alcohol use disorder. Rather, if used, they should be used in tandem with evidence-based cognitive-behavioral interventions known to be effective for alcohol use disorder harm reduction.
CITATIONS
Palpacuer, C., Duprez, R., Huneau, A., Locher, C., Boussageon, R., Laviolle, B., & Naudet, F. (2017). Pharmacologically controlled drinking in the treatment of alcohol dependence or alcohol use disorders: A systematic review with direct and network meta‐analyses on nalmefene, naltrexone, acamprosate, baclofen and topiramate. Addiction, In press. doi:10.1111/add.13974

