Policy change in Canada leads to increased pharmacy-based naloxone distribution among high-risk populations
Overdose deaths continue to rise in the United States and neighboring countries like Canada, largely driven by an increase in opioid overdoses. Naloxone can reverse an opioid overdose if given in enough time and at the appropriate dose. However, there are many barriers to accessing naloxone. In this study, researchers measured the impact of a policy change in Canada that removed some of these barriers.
WHAT PROBLEM DOES THIS STUDY ADDRESS?
Provisional data suggests that there were over 93,000 overdose deaths in the United States in 2020, a 32% increase from the previous year. The large majority of these overdose deaths involved opioids, and the COVID-19 pandemic has contributed to this recent rise. Opioid-related overdose deaths in Canada have also been on the rise. Several evidence-based interventions are available to decrease opioid overdose deaths, one of which is naloxone distribution. Naloxone is an opioid antagonist that can reverse an opioid overdose if given in enough time and at the appropriate dose. In the United States and Canada, naloxone distribution occurs through two primary mechanisms: dispensing from a pharmacy and distribution through a community program, such as an overdose education and naloxone distribution (OEND) program. Naloxone distribution has been repeatedly shown in simulation modelling studies to be one of the most effective interventions to decrease opioid overdose deaths. However, there are many barriers to obtaining and using naloxone, including cost, availability of user-friendly formulations, geographic disparities, stigma, and laws that restrict naloxone availability.
Research on the impact of existing policies in specific areas intended to reduce barriers to naloxone access can help not only to evaluate these current policies but also to inform future policies in areas where legislators are working to address the opioid overdose and related health crises. In this study, researchers examined the impact of a policy change in Ontario, Canada intended to reduce barriers to overall naloxone dispensing, with special consideration of how the policies affected certain high-risk populations such as those receiving opioid use disorder treatment.
HOW WAS THIS STUDY CONDUCTED?
This study used a population-based time series analysis of all residents in Ontario from July 2016 to March 2020 to examine monthly rates of pharmacy naloxone dispensing both before and after the policy change.
Implementation of the Ontario Naloxone Program for Pharmacies initiative in 2016 gave pharmacists the direct authority to provide injectable naloxone kits at no cost to all residents in the Canadian province. Although this program was shown to substantially increase the number of naloxone kits dispensed in Ontario, uptake was not uniform across the province and some high-risk groups, such as individuals on high doses of prescription opioids, had low uptake. The program was amended in March 2018 to include no-cost intranasal naloxone (known by the brand name Narcan) and removed the requirement for residents to produce a valid government health card to the dispensing pharmacist.
The research team was interested in measuring both overall dispensing of naloxone kits in the main analysis and dispensing among certain high-risk populations in a stratified analysis. The rate of naloxone dispensing was defined as the number of naloxone kits dispensed from Ontario pharmacies in a given month divided by the population of Ontario residents during that time period. For the stratified analysis, the population of Ontario was classified into four mutually exclusive groups:

The researchers hypothesized that there would be a change in naloxone dispensing immediately after the policy change in March 2018, so several statistical techniques were used to compare pharmacy-based naloxone distribution to Ontario residents before and after this time period.
WHAT DID THIS STUDY FIND?
The Program was effective in increasing naloxone distribution in the study period.
Overall, 446,690 naloxone kits were dispensed to 199,484 unique individuals (July 2016 to March 2020), with three-fourths of these individuals receiving naloxone kits after the policy changes in March 2018. Of the nearly 200,000 individuals that received a naloxone kit, 18% were on opioid agonist therapy, 19% were prescription opioid users, 8% had a prior history of opioid exposure, and 57% had no or an unknown opioid exposure history at the naloxone claim date. The monthly rate of pharmacy-based naloxone distribution increased from 1.9 to 111.5 kits per 100,000 people during the study period.
Naloxone distribution further increased after the policy change.
The overall rate of pharmacy-based naloxone distribution increased immediately after the policy changes in March 2018. Specifically, between February 2018 and May 2018 (two months before and after the policy changes) the monthly rate of dispensing increased from 55.6 to 91.8 kits per 100,000 people, an increase of 65%.
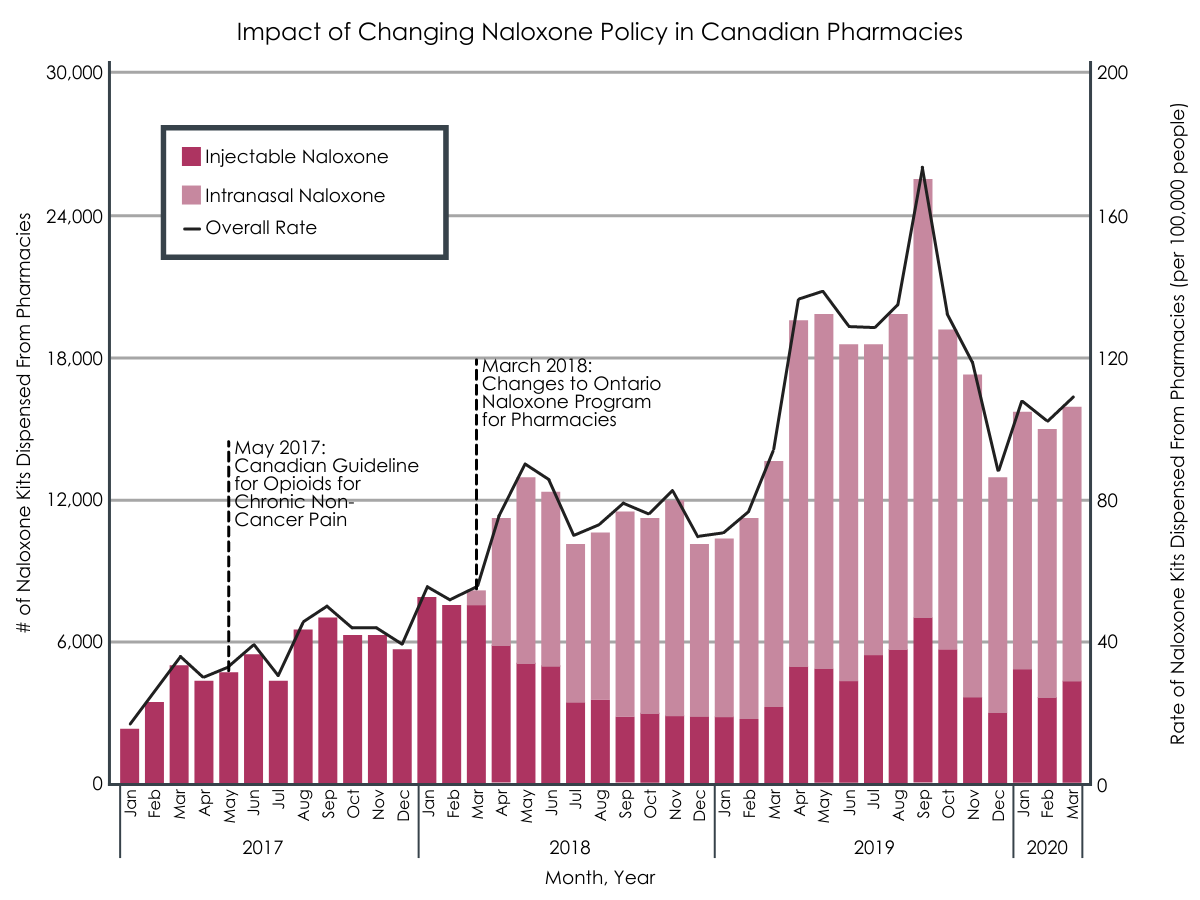
Increase in both number of naloxone kits dispensed, and in the rate at which they were dispensed, post-implementation of pharmaceutical naloxone program policy changes in March of 2018.
The increase in naloxone distribution was driven by dispensing to individuals at high risk for an opioid overdose.
There was a 115% increase in pharmacy-based naloxone distribution to individuals on opioid agonist therapy, a 98% increase to individuals on prescription opioids, and a 53% increase to individuals with a prior history of opioid exposure between February 2018 and May 2018. There was also a 63% increase in pharmacy-based naloxone distribution in urban regions of Ontario. There was no increase in rural regions and among those with no or an unknown history of opioid exposure.
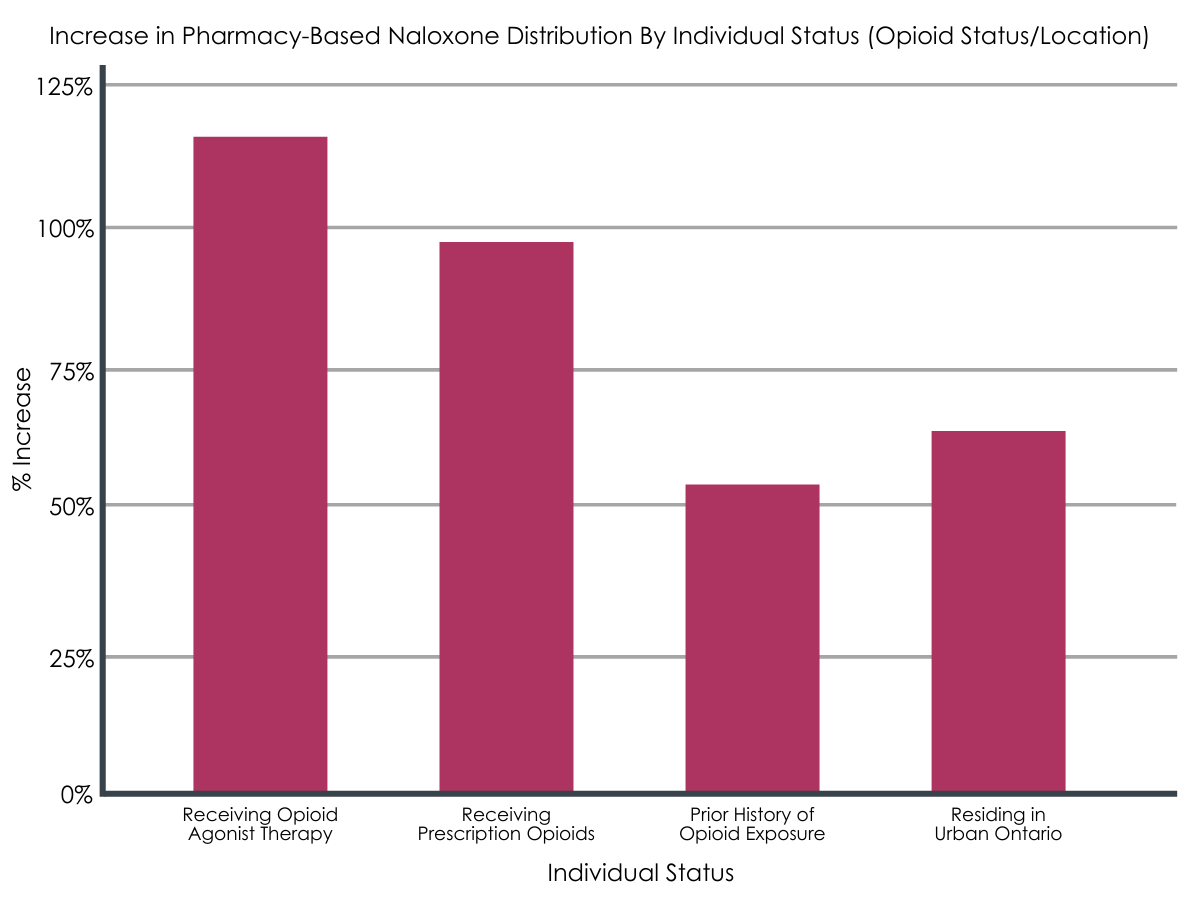
Statistically-significant increases in pharmacy-based naloxone distribution between individuals with various opioid status and geographic location within Ontario.
Intranasal naloxone was the preferred formulation.
In the last month of the study period (March 2020), the intranasal formulation represented 73% of the naloxone kits dispensed from pharmacies as part of the Ontario Naloxone Program for Pharmacies initiative.
Many individuals still showed identification to the dispensing pharmacist.
The percentage of individuals not showing a valid government health card to the dispensing pharmacist increased steadily since the policy change in March 2018. However, nearly two-thirds of individuals were still showing identification at the end of the study period in March 2020.
WHAT ARE THE IMPLICATIONS OF THE STUDY FINDINGS?
This study found that policy changes to the Ontario Naloxone Program for Pharmacies initiative, which made no-cost intranasal naloxone available to all residents of Ontario and removed the identification requirements to obtain naloxone, substantially increased pharmacy-based naloxone distribution. This increase was driven by populations at high-risk for an opioid overdose: individuals on medication treatment for opioid use disorder, individuals on prescription opioids, and individuals with a prior history of opioid exposure.
The findings of this study highlight the importance of policy interventions in removing barriers to naloxone accessibility. Expanding naloxone formulation options and removing a potentially stigmatizing requirement to show identification to access naloxone substantially increased pharmacy-based naloxone distribution in Ontario, Canada.
It is not clear which policy change was most influential in increasing pharmacy-based naloxone distribution, though the researchers speculate that access to intranasal naloxone was the primary driver. The naloxone policy landscape in the United States differs by state, and studies have shown that the enactment of certain naloxone access laws, such as co-prescribing laws (a mandate to prescribe naloxone along with certain doses of prescription opioids) and standing orders (allows patients to obtain naloxone at a pharmacy without a prescription), increased naloxone dispensing rates.
Of particular importance is that pharmacy-based naloxone distribution increased only among populations that are at high risk for an opioid overdose. The magnitude of this increase may be underestimated given that individuals who did not present a government health card, who are probably more likely to be in a high-risk group for opioid overdose, were not included in the group analyses.
This is significant given research that has shown naloxone distribution to be most effective for mortality reduction when targeted towards people who use drugs. However, the study also found that there was no increase among individuals with no, or an unknown, history, of opioid exposure – a large group that includes family and friends of someone who is at high risk for an opioid overdose. This is an important finding because a person experiencing an opioid overdose is unable to administer naloxone to themselves, so equipping family and friends who are most likely to witness an opioid overdose is a critical consideration when designing policy interventions to increase naloxone distribution in communities. While the study cannot tell us whether increased naloxone distribution reduced overdose death rates in Ontario, Canada, other population-level studies have shown that increased access to naloxone generally is associated with decreases in opioid-related overdose death rates.
A previous study on this initiative when only injectable naloxone was available showed that, although distribution increased overall, pharmacy-based naloxone dispensing did not increase among individuals on prescription opioids. This finding suggests that these individuals may not feel comfortable using an injectable formulation of naloxone or would prefer to access naloxone anonymously (i.e., without having to show health card identification, similar to a health insurance card in the United States) for fear of having their prescription opioids reduced or discontinued. In addition, these types of individuals may not feel comfortable accessing naloxone through a community program, such as a harm reduction site. Expanding access to a more user-friendly formulation of naloxone and allowing anonymous access to naloxone seems to have increased the rate of naloxone dispensing to this high-risk group, which has been found to have a low perceived risk of opioid overdose.
- LIMITATIONS
-
- This study did not have a control group. Therefore, influences other than the policy change examined in this study may have increased pharmacy-based naloxone distribution, though the researchers did identify and account statistically for a change in national guidelines for chronic non-cancer pain that supported provision of naloxone.
- This study did not capture all channels of naloxone distribution in Ontario, such as distribution through overdose education and naloxone distribution programs.
- Due to differences in health policy between Canada and other countries, generalizability of the study findings is limited, especially for the United States where there are more barriers to health care access.
- As mentioned above, those who did not present an identification card after the policy change were not included in the stratified group analyses.
BOTTOM LINE
Findings from this study suggest that policy changes that remove barriers to naloxone accessibility at the pharmacy level, specifically making a more user-friendly formulation available and removing the requirement for showing identification to the dispensing pharmacist, was associated with increased pharmacy-based naloxone distribution in Ontario, Canada. This increase, overall, was driven by increased distribution among populations at high-risk for an opioid overdose: individuals on medication treatment for opioid use disorder, individuals on prescription opioids, and individuals with a prior history of opioid exposure.
- For individuals and families seeking recovery: Naloxone is a life-saving medication with no misuse liability that reverses the effect of an opioid overdose if given in enough time and at an appropriate dose. It should be carried by all individuals using opioids, including those on high doses of prescribed opioids for chronic pain, and their family and friends. Policy interventions have immense potential to increase naloxone accessibility on a broad scale and differ by state. Encouraging both local and state policymakers and community organizations to support naloxone availability initiatives could potentially save lives. Please see this resource to find about more about naloxone policies in your state.
- For treatment professionals and treatment systems: Naloxone is a life-saving medication with no misuse liability that reverses the effect of an opioid overdose if given in enough time and at an appropriate dose. It should be carried by all individuals using opioids, including those on high doses of prescribed opioids for chronic pain, and their family and friends. Stocking naloxone by treatment professionals and providing it to clients might reduce barriers to access. Clients who are at risk for an opioid overdose are most likely to benefit from increased naloxone access, including those on medication treatment for opioid use disorder, those on high doses of prescription opioids, and those who are actively using opioids, methamphetamine, or cocaine. Policy interventions have immense potential to increase naloxone accessibility on a broad scale and differ by state. Encouraging both local and state policymakers and community organizations to support naloxone availability initiatives could potentially save lives. Please see this resource to find about more about naloxone policies in your state.
- For scientists: Findings from this study show a substantial increase in pharmacy-based naloxone distribution following policy changes to remove barriers to this life-saving medication, although there was no control group and the data used did not capture all of the naloxone being distributed to communities. Future research will be better informed by capturing naloxone distribution from the two primary mechanisms of dispensing from a pharmacy and distribution through a community program. In addition, the policy changes did not increase naloxone distribution to people that were not classified as high risk, which is a heterogeneous group that includes family and friends of someone who is at risk for an opioid overdose. Further research exploring barriers of naloxone access in this group is warranted. In the United States, the effect of naloxone access laws on mortality is mixed and may reflect methodological issues that could be the subject of future research. Research on naloxone policies may be especially feasible in Canada given access to extensive health data on all residents in the country.
- For policy makers: Naloxone is a life-saving medication with no misuse liability that reverses the effect of an opioid overdose if given in enough time and at an appropriate dose. It should be carried by all individuals using opioids, including those on high doses of prescribed opioids for chronic pain, and their family and friends. However, accessing this opioid overdose antidote is difficult for many, in large part because of restrictive laws regulating its distribution, cost barriers, and limited access to user-friendly formulations of naloxone. Policy interventions have immense potential to remove these barriers and increase naloxone accessibility on a broad scale. The policy landscape for naloxone access differs by state. Statewide standing orders or direct prescriptive authority to pharmacists, where an individual can be dispensed naloxone without a prescription, have been proven to increase naloxone distribution in communities. Co-prescribing laws that mandate prescribing naloxone along with high doses of prescription opioids increases naloxone distribution to individuals at high risk for an opioid overdose who would not normally consider naloxone. Please see this resource to find about more about naloxone policies in your state.
CITATIONS
Antoniou, T., Martins, D., Campbell, T., Tadrous, M., Munro, C., Leece, P., Mamdani, M., Juurlink, D.N., Gomes, T. (2021). Impact of policy changes on the provision of naloxone by pharmacies in Ontario, Canada: A population-based time-series analysis. Addiction, 116(6), 1514-1520. DOI: 10.1111/add.15324.

