Should medication dose guidelines vary by country? An evaluation of buprenorphine dose and treatment compliance in India
Buprenorphine (often prescribed with naloxone and known by the brand name Suboxone) dosing guidelines in India suggest effective opioid use disorder treatment of the Indian population at doses of 4 to 8mg. However, it is unclear if buprenorphine dose influences treatment adherence among the Indian population. Consistent with studies of American patients, this study suggests that treatment adherence is much more likely at higher buprenorphine doses.
WHAT PROBLEM DOES THIS STUDY ADDRESS?
Opioid use disorder is a global problem, with about 27 million people suffering from it worldwide. In India, the prevalence of illicit opioid use is shown to be twice that of the global average. Not surprisingly, treatment seeking for opioid use problems has also risen in India. Though studies have helped us better understand treatment efficacy in North America and Europe, there is an ongoing need to identify effective opioid use disorder treatment regimens in other regions affected by the opioid crisis. Buprenorphine, a medication for treating opioid use disorder, is prescribed in generally lower doses in India than what is typically suggested per international guidelines. In the United States, a dose of at least 8mg is the standard recommendation, and studies suggest that higher doses (i.e., 16mg) can decrease the likelihood of treatment drop-out. Empirical studies conducted in India have resulted in different dosing guidelines, suggesting effective treatment of the Indian population at doses of 4 to 8mg. However, it is unclear whether these lower doses of buprenorphine influence short-term adherence among the Indian population. The current study aimed to address this gap in the field by assessing the influence of dose on medication adherence after 6 weeks of buprenorphine treatment.
HOW WAS THIS STUDY CONDUCTED?
The authors conducted a longitudinal observational study of 89 patients who were starting buprenorphine (often prescribed with naloxone and known by the brand name Suboxone) treatment for opioid use disorder (i.e., opioid dependence, as diagnosed with the ICD-10), and the factors associated with their treatment noncompliance. Patients were evaluated just prior to starting medication treatment (baseline), and again six weeks later (follow-up). The authors collected information regarding substance use histories, neuropsychiatric disorders (Mini International Neuropsychiatric Interview Plus) and their severity (Addiction Severity Index-Lite), Social Support (Multi-Dimensional Scale of Perceived Social Support), opioid craving (Heroin Craving Questionnaire – Short Form). Drug testing was done at baseline and follow-up to test for opioids and buprenorphine use. Participants were considered treatment adherent if they reported taking at least 90% of their prescribed buprenorphine dose during treatment. Non-compliant patients included individuals who took less than 90% of their prescribed dose and those who did not show up for the follow-up assessment. As part of treatment, all participants also received psychoeducation, motivation enhancement, and relapse prevention counseling.
Participant were primarily male (94%) and employed (78%), with less than 12 years of education (60%) and an average age of 33 years old. 32% of individuals had a comorbid psychiatric disorder. Participants reported prescription opioids (53%), illicit opioids (38%), or both (9%) as their primary problem substance. 35% of individuals reported injection drug use at least once in their lifetime. These measures did not significantly differ between the treatment adherent and non-adherent groups.
WHAT DID THIS STUDY FIND?
At follow-up, 67% of patients were adherent to their medication, while 33% were non-adherent. Participants’ average buprenorphine dose was 6.7mg. The treatment adherent group received a higher average dose of buprenorphine (7.7mg) than the non-adherent group (4.3mg). Odds of treatment non-adherence were three times higher in patients who received a buprenorphine dose of 6mg or less (0.63) when compared to those who received more than 6mg (0.20). Of note, this comparison did not control for any factors that differed between the groups; in this case, those who were adherent were more likely to report illicit opioids as their primary drug than those who were non-adherent to their medication (Non-Adherent group: 22% vs. Adherent group: 45%). Rates of non-adherence were 17% among individuals who received a dose greater than 6mg, and 39% among individuals who received a dose of 6mg or less.
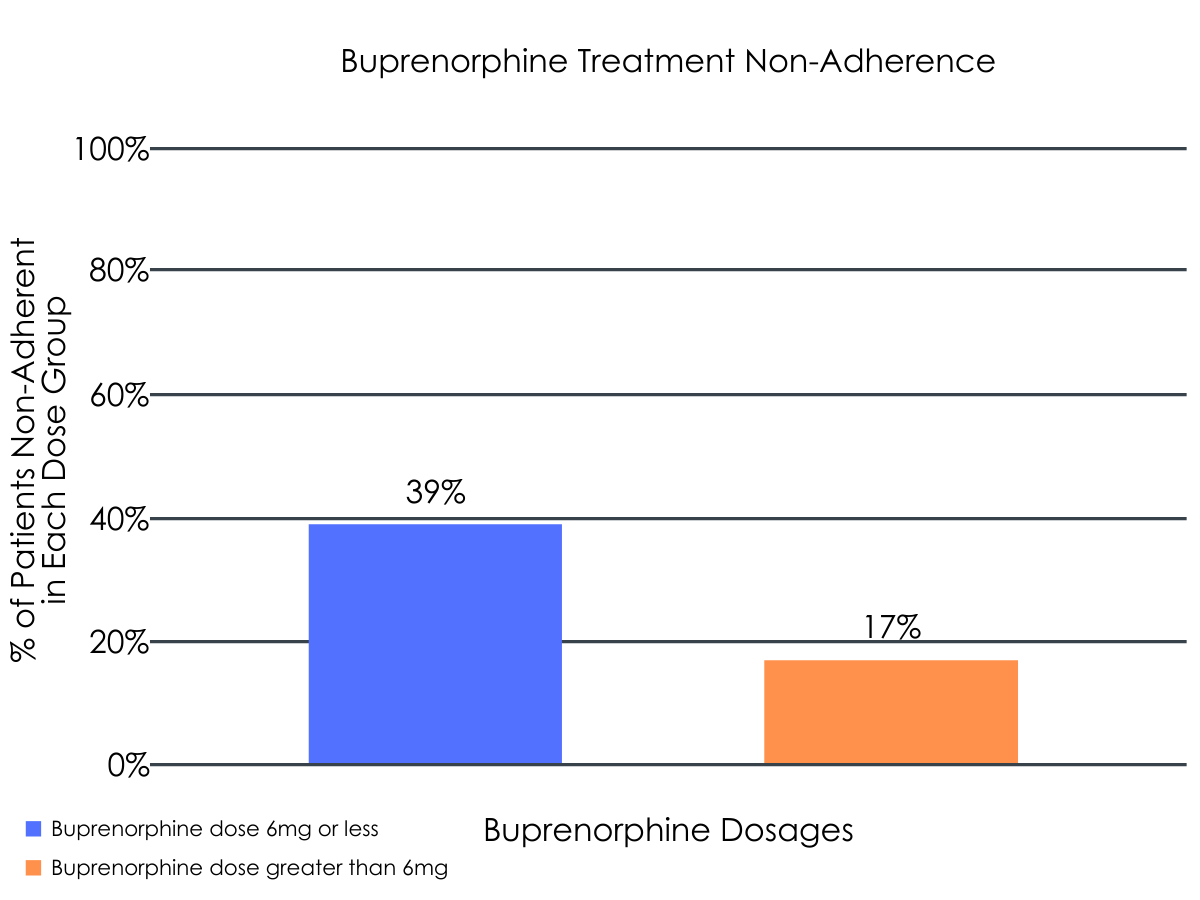
Figure 1.
WHAT ARE THE IMPLICATIONS OF THE STUDY FINDINGS?
Cultural sensitivity and awareness of the potential for biological and physiological differences according to nationality are important factors with regard to opioid use disorder treatment. Evidence suggests that opioid use disorder patients in India effectively respond to buprenorphine treatment at lower doses, ranging from 4-8mg. Though the reasons for effective treatment at lower doses is not yet understood in Indian patients, researchers speculate differences in culture, substance use patterns, and physiology may play a role. Rates of opioid abstinence and withdrawal demonstrated among Indian patients treated with these lower doses are shown to be comparable to international studies implementing higher doses. However, this study mirrors the results of investigations in the United States, suggesting that higher doses of buprenorphine can help boost compliance in the early stages of medication treatment. A 16mg buprenorphine dose is considered sufficiently high to boost adherence in the United States, where guidelines recommend a dose of at least 8mg is prescribed to patients. This study suggests that doses greater than 6mg might substantially increase the odds of treatment compliance among patient populations native to India, where guidelines recommend a minimum dose of at least 4mg. Given the Indian population’s differential response to buprenorphine dosing in general (i.e., requiring lower doses for a beneficial therapeutic effect), but the comparable observation of better treatment compliance at higher buprenorphine doses, longitudinal research is needed to identify the optimal dose necessary for incurring the most robust compliance effects. It is important to note, however, that this study’s adherence outcomes may be confounded by disorder severity, with the adherent group being more likely to use illicit opioids than the non-adherent group. To disentangle the direct effects of dose on adherence, additional research that controls for baseline differences and incorporates larger samples is needed. Identifying the factors that play a role in treatment response across different cultures (e.g., metabolism, local substance use preferences and patterns, etc.) will ultimately help clinicians develop treatment plans that cater to different opioid use disorder populations.
- LIMITATIONS
-
- This study’s follow-up occurred six weeks after the start of medication treatment. Therefore, it is unclear if dose had a protracted impact on treatment compliance beyond this timeframe. It is also unclear whether dose affects other aspects of treatment and recovery (e.g., well-being, happiness), and more research is needed to better understand these issues across different cultures.
- The non-compliant group included individuals who did not show up for the follow-up assessment, which has the potential to impact outcomes. Although the other demographic and substance use variables did not significantly differ between compliant and non-compliant patients, the sample size was small and raw data reported by the authors suggest the need for additional research with larger patient samples, controlling for baseline participant characteristics.
- The sample size was too small to measure the influence of dose as a continuous measure and dose may have varied widely among patients. The authors therefore used a median split of the sample (those receiving more than 6mg vs. those receiving 6mg or less) to evaluate the odds of treatment compliance. More research is needed to determine the optimal dose for enhancing compliance.
BOTTOM LINE
- For individuals and families seeking recovery: This study looked at the effects of dose on treatment compliance among opioid use disorder patients in India. Similar to studies conducted in America, the authors found that the likelihood of treatment adherence was significantly higher when patients were given a higher dose of buprenorphine. However, optimal response to buprenorphine may be achieved at lower doses in Indian patients (6mg) and higher doses in American patients (16mg). Although the reasons for this are not yet understood, dose response differences highlight the importance of culture in an individual’s treatment plan. Given that dose is an important factor in a treatment plan and response varies from person to person, individuals and families seeking buprenorphine treatment are encouraged to discuss their treatment plan with their providers to receive a medication dose and regimen that promotes successful recovery.
- For treatment professionals and treatment systems: This study evaluated the impact of dose on treatment compliance among opioid use disorder patients in India. The authors found that the odds of treatment non-adherence were three times higher if patients who received a buprenorphine dose of 6mg or less. This study mirrors the results of American investigations, showing that higher doses of buprenorphine enhance treatment compliance. However, opioid use disorder patients in America experience the benefits of buprenorphine treatment at a dose of at least 16mg, highlighting cultural differences in medication response. Therefore, being aware of potential cultural differences in dose response and the importance of prescribing a sufficiently high dose during the early stages of treatment can help inform providers when formulating individualized treatment plans.
- For scientists: This study evaluated the impact of dose and other descriptive factors on treatment compliance among opioid use disorder patients in India. The authors found that the odds of treatment non-adherence were three times higher in patients who received a buprenorphine dose of 6mg or less. Additional research is needed to characterize opioid use disorder treatment response and recovery outcomes with regard to race, ethnicity, and culture among individuals residing in different regions of the world. Understanding the ideal pharmacotherapy dose for optimal treatment response and the individual characteristics that influence this dose-response curve will ultimately help address an international opioid problem. Identifying the biological, physiological, and environmental factors contributing to differential pharmacotherapy response will be essential for facilitating evidence-based treatment, inclusive of all opioid use disorder patients.
- For policy makers: This study found that a buprenorphine dose of 6mg or less can significantly hinder treatment compliance among opioid use disorder patients in India. Alternatively, evidence suggests that opioid use disorder patients in America effectively respond to buprenorphine at a dose of 16mg. These outcomes highlight the importance of understanding cross-cultural differences in opioid use disorder treatment and response to medication. Given that dose effects vary from person to person, additional funding for cross cultural research is needed to better understand how different cultures respond to particular treatment plans. Identifying the factors that contribute to differential medication response can inform optimal individualized treatment plans, and ultimately help to address an international opioid problem.
CITATIONS
Muruganandam, P., Shukla, L., Sharma, P., Kandasamy, A., Chand, P., & Murthy, P. (2019). ‘Too little dose–too early discontinuation?’—Effect of buprenorphine dose on short term treatment adherence in opioid dependence. Asian Journal of Psychiatry, 44, 58-60 doi: 10.1016/j.ajp.2019.07.030

