Scientists Look Into a Promising New Pharmacological Treatment for Alcohol Use Disorder
As researchers continue to evaluate the efficacy of medications for the treatment of alcohol use disorder, mixed results from trials can make it difficult to determine if the medication itself is ineffective or if further research is needed to adjust dosing.
WHAT PROBLEM DOES THIS STUDY ADDRESS?
Baclofen, a medication used to treat muscle spasms may also have a therapeutic effect in patients with alcohol use disorder since it acts on the GABA-B receptor, part of the system involved in the development of alcohol dependence (see here).
However, previous studies examined the efficacy of low dosages of baclofen which may impact results due to its low ability to cross the blood brain barrier.
In other words, higher doses may be needed to reach a therapeutic dose in some individuals. This randomized controlled trial (RCT) addresses this issue by testing the efficacy of high doses of baclofen with dose adjusted for each participant based on their tolerability of the medication.
HOW WAS THIS STUDY CONDUCTED?
This 24 week RCT included adult patients meeting DSM-IV-TR and ICD-10 criteria for alcohol dependence (i.e., addiction to alcohol) recruited from the inpatient and outpatient units at a hospital in Berlin, Germany.
Participants reported heavy drinking (i.e., ≥5 drinks per day for men and ≥4 drinks per day for women) at least twice a week with an intake of 21 drinks or more per week for men and 14 drinks or more per week for women prior to completing detoxification. Participants also needed to have last consumed alcohol 7 to 21 days before randomization.
Fifty-six participants were randomized to receive baclofen (n = 28) or placebo (n = 28).
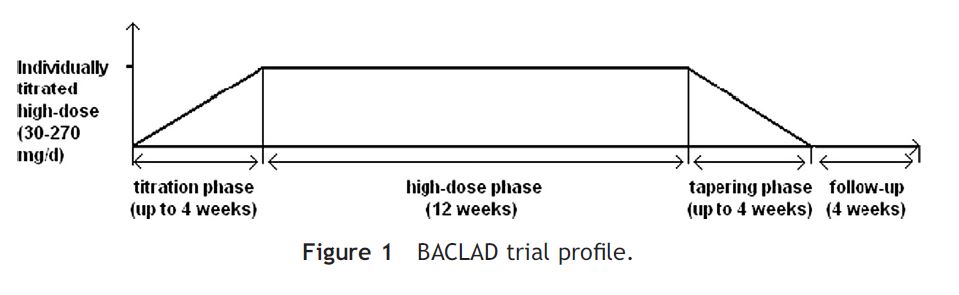
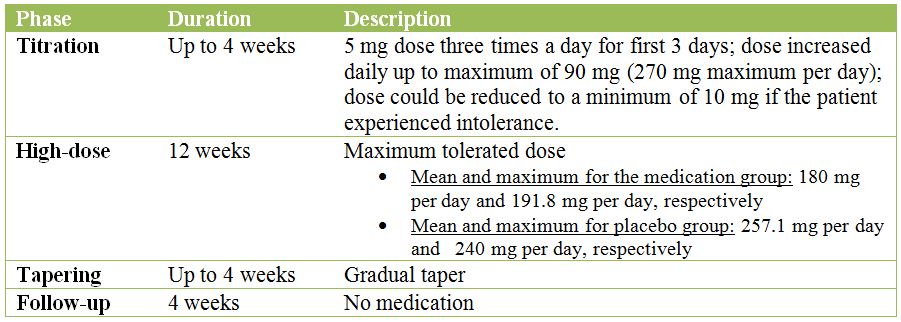
The four phases described in detail below:
WHAT DID THIS STUDY FIND?
NOTABLY FROM THE STUDY:
- Of 28 patients in the baclofen group, 10 reached the maximum dose of 270 mg per day
- The mean dose during the high-dose phase was 180 mg per day
- Significantly more participants in the baclofen group (68%) remained abstinent during the high-dose phase than the placebo group (24%)
- Cumulative abstinence duration was also significantly longer during this period for baclofen participants (68 days) compared to placebo (52 days)
- Significantly more patients maintained abstinence in the baclofen group compared to placebo (43% versus 14%) (When analyzing the entire medication period, all 4 phases combined)
- The baclofen group had a higher average duration of cumulative abstinence (83 days) than placebo (67 days), but this difference was not significant
- Compared to individuals in the placebo group, the likelihood (using the hazard ratio; learn more about hazard ratios here) of relapsing was 70% lower for participants in the baclofen group during the high-dose phase and 50% lower during the entire medication period
Significantly more patients maintained abstinence in the baclofen group.
As the first RCT evaluating high-dose baclofen, this study provides evidence for this medication’s ability to promote abstinence in alcohol dependent patients. Additionally, the medication was well-tolerated; there were no severe adverse events and side effects were minimal with fatigue occurring in only two patients. Baclofen may be a promising option for medication-assisted treatment in the future.
WHY IS THIS STUDY IMPORTANT
There are currently four Federal drug Administration (FDA) approved medications for the treatment of alcohol use disorder:
- oral naltrexone
- injectable naltrexone
- disulfiram
- acamprosate
with an additional one (nalmefene) is approved for use in Europe.
While there are multiple options available to patients, studies show a reliable but modest impact on alcohol outcomes. This presents an opportunity for further development of pharmacological interventions.
This study from Europe supports the benefits of baclofen but is the first of its kind. Based on case reports, France approved dosages up to 300 mg per day for alcohol-dependent patients. More research, including studies in the U.S., is needed before it can be approved for use and widespread adoption.
- LIMITATIONS
-
- The main limitation of this study was its small sample size; the study was underpowered to assess cumulative abstinence duration. Having a larger sample (i.e., more participants) makes it easier to detect significant effects.
- Also, the sample was recruited from a single site in Berlin, Germany which raises issues of generalizability.
NEXT STEPS
More RCTs in different populations with larger sample sizes are needed to substantiate these results. Studies also need to identify an optimal biological dose and determine if there is potential for misuse. Baclofen should also be compared to other active medications that are already FDA approved such as naltrexone. Other outcomes such as reductions in heavy drinking should also be examined.
BOTTOM LINE
- For individuals & families seeking recovery: While evidence from this trial is promising, baclofen is not approved for treating alcohol use disorder in the U.S. Speak with an addiction professional about other medications or behavioral treatment options.
- For scientists: More RCTs with baclofen are needed to substantiate the results of this study.
- For policy makers: Baclofen may be a promising treatment option for alcohol use disorder. Funding for research investigating the benefits of this medication is warranted.
- For treatment professionals and treatment systems: Baclofen may be an option for patients in the future.
CITATIONS
Muller, C. A., Geisel, O., Pelz, P., Higl, V., Kruger, J., Stickel, A., . . . Heinz, A. (2015). High-dose baclofen for the treatment of alcohol dependence (BACLAD study): A randomized, placebo-controlled trial. Eur Neuropsychopharmacol, 25(8), 1167-1177. doi:10.1016/j.euroneuro.2015.04.002

